An automated method for analysis of microcirculation videos for accurate assessment of tissue perfusion - PubMed (original) (raw)
An automated method for analysis of microcirculation videos for accurate assessment of tissue perfusion
Sumeyra U Demir et al. BMC Med Imaging. 2012.
Abstract
Background: Imaging of the human microcirculation in real-time has the potential to detect injuries and illnesses that disturb the microcirculation at earlier stages and may improve the efficacy of resuscitation. Despite advanced imaging techniques to monitor the microcirculation, there are currently no tools for the near real-time analysis of the videos produced by these imaging systems. An automated system tool that can extract microvasculature information and monitor changes in tissue perfusion quantitatively might be invaluable as a diagnostic and therapeutic endpoint for resuscitation.
Methods: The experimental algorithm automatically extracts microvascular network and quantitatively measures changes in the microcirculation. There are two main parts in the algorithm: video processing and vessel segmentation. Microcirculatory videos are first stabilized in a video processing step to remove motion artifacts. In the vessel segmentation process, the microvascular network is extracted using multiple level thresholding and pixel verification techniques. Threshold levels are selected using histogram information of a set of training video recordings. Pixel-by-pixel differences are calculated throughout the frames to identify active blood vessels and capillaries with flow.
Results: Sublingual microcirculatory videos are recorded from anesthetized swine at baseline and during hemorrhage using a hand-held Side-stream Dark Field (SDF) imaging device to track changes in the microvasculature during hemorrhage. Automatically segmented vessels in the recordings are analyzed visually and the functional capillary density (FCD) values calculated by the algorithm are compared for both health baseline and hemorrhagic conditions. These results were compared to independently made FCD measurements using a well-known semi-automated method. Results of the fully automated algorithm demonstrated a significant decrease of FCD values. Similar, but more variable FCD values were calculated using a commercially available software program requiring manual editing.
Conclusions: An entirely automated system for analyzing microcirculation videos to reduce human interaction and computation time is developed. The algorithm successfully stabilizes video recordings, segments blood vessels, identifies vessels without flow and calculates FCD in a fully automated process. The automated process provides an equal or better separation between healthy and hemorrhagic FCD values compared to currently available semi-automatic techniques. The proposed method shows promise for the quantitative measurement of changes occurring in microcirculation during injury.
Figures
Figure 1
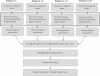
Schematic of the proposed methodology. Proposed methodology is summarized in Figure 1. Stabilization of the videos is not included in the schematics. After stabilization, the weighted mean of five consecutive frames is calculated and the preprocessing and segmentation algorithms are applied on the mean frame. Averaging more than five frames results in “over-averaging” phenomena that would eliminate some of the important features important for segmentation. Experimentally, our results indicate that the 5-frame approach provides the best results. After the segmentation, the segmented frames are combined together to generate one single binary image and calculate Functional Capillary Density from this binary image.
Figure 2
Detailed diagram of pre-processing and vessel segmentation. Figure 2 provides a detailed diagram of preprocessing and segmentation steps for different threshold levels. It starts with the averaged frame. For 10 different threshold levels, the parameters of CLAHE (Contrast Limited Adaptive Histogram Equalization) and median filter vary throughout the process. For the first threshold level, the window size of CLAHE is kept small. Median filter is applied right after histogram equalization with a small filter size such as 3 × 3. Median filtering is followed by image adjustment. The preprocessed image is converted to binary image using the first threshold level. Euclidean Distance Transform (EDT) is calculated for the binary image. Diameter and angle parameters are extracted from EDT and with the addition of contrast ratio; three parameters are used to determine if a pixel belongs to a vessel.
Figure 3
Method of validating vessel pixels. A vessel candidate pixel is labeled as p in Figure 3. The output of EDT is used to find the nearest background pixel to p, b p. For each of the 24 neighboring pixels in the 5 × 5 neighborhood around p, _n_1 − _n_24, the nearest background pixel is found, b n, and used to calculate the diameter, angle and contrast ratio values. The b n1-24 having the greatest distance from b p is considered the opposite background pixel, b max, and the distance is the diameter, d, of the vessel. If d is less than P d then the angle, θ, is calculated between b p, p and b max and is used to validate the distance by ensuring that b p and b max are on opposite sides of the vessel (θ must be greater than P θ). Finally, the contrast ratio between p and b max is calculated and if greater than P c, the candidate pixel is considered a valid vessel pixel. Since the found vessels lie along the center of the actual vessel, the vessel must be reconstructed using the found diameter and pixel locations.
Figure 4
An example frame from a healthy subject. An example frame from a sublingual microcirculatory video captured from a healthy baseline subject is presented.
Figure 5
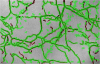
Segmented active capillaries of the frame in Figure4**.** The result of the proposed algorithm highlights all active capillaries from the original frame in Figure 4.
Figure 6
Example of hemorrhage subject video source. An original frame from sublingual microcirculatory video of a hemorrhage subject.
Figure 7
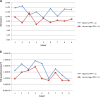
FCD results calculated from heavily edited AVA. The FCD values calculated from heavily edited AVA for both healthy baseline and hemorrhage conditions are displayed. The healthy condition FCD values are labeled as baseline. The change in FCD values during hemorrhage is not consistent. a: FCD results (area based) from heavily edited AVA show inconsistent separation between the healthy (baseline, PPV = 1) and hemorrhagic (PPV < 1) cases. b: FCD results (length based) from heavily edited AVA.
Figure 8
FCD results calculated using the proposed algorithm (MCA). The FCD values calculated using the proposed algorithm for both healthy baseline and hemorrhage conditions are displayed. The healthy condition FCD values are labeled as baseline. The decrease in FCD values for each subject during hemorrhagic is obvious in the provided figure. a: FCD results (area based) from the proposed automated system show better and consistent separation between healthy (baseline, PPV = 1) and hemorrhagic (PPV < 1) cases. b: FCD results (length based) from the proposed automated system show good separation, but demonstrate the problem with length based FCD calculation where vessel width is not taken into consideration as it is with area based FCD.
Figure 9
Overlay of proposed automated method onto heavily edited AVA results showing a high degree of similarity. Results from proposed automated method (green) superimposed over results from heavily edited AVA (red/black). Proposed method returns results 60 to 120 times faster than manual editing in AVA (20 seconds vs. 20-40 minutes).
Figure 10
Frame of video used for analysis in Figure9**.** An example frame of the video used to generate Figure 9 in both MCA and heavily edited AVA.
Figure 11
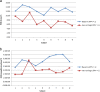
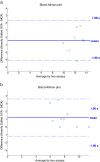
Bland-Altman plots showing validity of fully-automated MCA as a measurement tool vs. current “gold standard” of heavily edited AVA (baseline (a) and hemorrhagic (b). a: Bland-Altman plot showing correlation between heavily edited AVA and fully-automated MCA for baseline (PPV = 1) subjects. b: Bland-Altman plot showing correlation between heavily edited AVA and fully-automated MCA for hemorrhagic (PPV < 1) subjects.
Similar articles
- An automated method for analysis of flow characteristics of circulating particles from in vivo video microscopy.
Eden E, Waisman D, Rudzsky M, Bitterman H, Brod V, Rivlin E. Eden E, et al. IEEE Trans Med Imaging. 2005 Aug;24(8):1011-24. doi: 10.1109/TMI.2005.851759. IEEE Trans Med Imaging. 2005. PMID: 16092333 - An integrated system for the segmentation of atherosclerotic carotid plaque ultrasound video.
Loizou C, Petroudi S, Pantziaris M, Nicolaides A, Pattichis C. Loizou C, et al. IEEE Trans Ultrason Ferroelectr Freq Control. 2014 Jan;61(1):86-101. doi: 10.1109/TUFFC.2014.6689778. IEEE Trans Ultrason Ferroelectr Freq Control. 2014. PMID: 24402898 - A rain pixel recovery algorithm for videos with highly dynamic scenes.
Jie Chen, Lap-Pui Chau. Jie Chen, et al. IEEE Trans Image Process. 2014 Mar;23(3):1097-104. doi: 10.1109/TIP.2013.2290595. Epub 2013 Nov 12. IEEE Trans Image Process. 2014. PMID: 24240000 - Conjunctival microcirculation in ocular and systemic microvascular disease.
Asiedu K, Krishnan AV, Kwai N, Poynten A, Markoulli M. Asiedu K, et al. Clin Exp Optom. 2023 Sep;106(7):694-702. doi: 10.1080/08164622.2022.2151872. Epub 2023 Jan 15. Clin Exp Optom. 2023. PMID: 36641840 Review. - History of the cutaneous microcirculation from antiquity to modern times.
Jung F. Jung F. Clin Hemorheol Microcirc. 2024;86(1-2):29-50. doi: 10.3233/CH-248001. Clin Hemorheol Microcirc. 2024. PMID: 38363606 Review.
Cited by
- Sidestream dark field images of the microcirculation: intra-observer reliability and correlation between two semi-quantitative methods for determining flow.
Petersen SM, Greisen G, Hyttel-Sorensen S, Hahn GH. Petersen SM, et al. BMC Med Imaging. 2014 May 6;14:14. doi: 10.1186/1471-2342-14-14. BMC Med Imaging. 2014. PMID: 24885423 Free PMC article. - Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine.
Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, Pinsky MR, Artigas A, Teboul JL, Reiss IKM, Aldecoa C, Hutchings SD, Donati A, Maggiorini M, Taccone FS, Hernandez G, Payen D, Tibboel D, Martin DS, Zarbock A, Monnet X, Dubin A, Bakker J, Vincent JL, Scheeren TWL; Cardiovascular Dynamics Section of the ESICM. Ince C, et al. Intensive Care Med. 2018 Mar;44(3):281-299. doi: 10.1007/s00134-018-5070-7. Epub 2018 Feb 6. Intensive Care Med. 2018. PMID: 29411044 - MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy.
Hilty MP, Guerci P, Ince Y, Toraman F, Ince C. Hilty MP, et al. Commun Biol. 2019 Jun 19;2:217. doi: 10.1038/s42003-019-0473-8. eCollection 2019. Commun Biol. 2019. PMID: 31240255 Free PMC article. - A guide to human in vivo microcirculatory flow image analysis.
Massey MJ, Shapiro NI. Massey MJ, et al. Crit Care. 2016 Feb 10;20:35. doi: 10.1186/s13054-016-1213-9. Crit Care. 2016. PMID: 26861691 Free PMC article. - Deep learning and computer vision techniques for microcirculation analysis: A review.
Helmy M, Truong TT, Jul E, Ferreira P. Helmy M, et al. Patterns (N Y). 2022 Dec 1;4(1):100641. doi: 10.1016/j.patter.2022.100641. eCollection 2023 Jan 13. Patterns (N Y). 2022. PMID: 36699745 Free PMC article. Review.
References
- Cĕrný V, Turek Z, Pařízková R. Orthogonal polarization spectral imaging: a review. Physiol Res. 2007;56:141–147. - PubMed
- Hebbel R, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease; inflammation and chronic vasculopathy. Microcirculation. 2004;11:129–151. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical