Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation - PubMed (original) (raw)
Novel bilobe components in Trypanosoma brucei identified using proximity-dependent biotinylation
Brooke Morriswood et al. Eukaryot Cell. 2013 Feb.
Abstract
The trypanosomes are a family of parasitic protists of which the African trypanosome, Trypanosoma brucei, is the best characterized. The complex and highly ordered cytoskeleton of T. brucei has been shown to play vital roles in its biology but remains difficult to study, in large part owing to the intractability of its constituent proteins. Existing methods of protein identification, such as bioinformatic analysis, generation of monoclonal antibody panels, proteomics, affinity purification, and yeast two-hybrid screens, all have drawbacks. Such deficiencies-troublesome proteins and technical limitations-are common not only to T. brucei but also to many other protists, many of which are even less well studied. Proximity-dependent biotin identification (BioID) is a recently developed technique that allows forward screens for interaction partners and near neighbors in a native environment with no requirement for solubility in nonionic detergent. As such, it is extremely well suited to the exploration of the cytoskeleton. In this project, BioID was adapted for use in T. brucei. The trypanosome bilobe, a discrete cytoskeletal structure with few known protein components, represented an excellent test subject. Use of the bilobe protein TbMORN1 as a probe resulted in the identification of seven new bilobe constituents and two new flagellum attachment zone proteins. This constitutes the first usage of BioID on a largely uncharacterized structure, and demonstrates its utility in identifying new components of such a structure. This remarkable success validates BioID as a new tool for the study of unicellular eukaryotes in particular and the eukaryotic cytoskeleton in general.
Figures
Fig 1
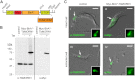
Characterization of Myc-BirA*-TbMORN1-expressing cells. (A) Schematic drawing showing cloning of the TbMORN1 open reading frame into the modified pLew100_Myc_BirA* plasmid for tetracycline-inducible expression. (B) Myc-BirA*-TbMORN1 is tightly and inducibly expressed. 29-13 cells stably transfected with the pLew100_Myc_BirA*-TbMORN1 plasmid were incubated in the presence or absence of 10 ng/ml tetracycline. Whole-cell lysates were analyzed by immunoblots with either anti-TbMORN1 or anti-Myc antibodies. In untransfected (control) cells and uninduced cells, only the endogenous TbMORN1 (40 kDa) is detected. In the presence of tetracycline, an additional approximately 75 kDa polypeptide was detected by both antibodies. (C) Myc-BirA*-TbMORN1 recapitulates the localization and morphology of the endogenous protein. Untransfected (control) cells or Myc-BirA*-TbMORN1-expressing cells were analyzed by immunofluorescence assay. Intact (panels i and ii) and detergent-extracted (panels iii and iv) cells were labeled with anti-TbMORN1 or anti-Myc antibodies. The bilobe structure is indicated (white arrows, area shown enlarged in insets). Scale bars, 2 μm.
Fig 2
Biotinylation by Myc-BirA*-TbMORN1 in vivo. Myc-BirA*-TbMORN1 cells were incubated with excess biotin in the presence or absence of 10 ng/ml tetracycline (Tet). (A and B) Immunofluorescence assay of intact cells labeled with anti-Myc antibodies and fluorescently conjugated streptavidin. In the presence of tetracycline (A), the two labels strongly overlapped. The bilobe (arrows, area enlarged in insets) was clearly visible. In the absence of tetracycline (B), only endogenously biotinylated proteins were labeled as a background signal. (C and D) Immunofluorescence assay of detergent-extracted cells labeled as in panels A and B. In the presence of tetracycline (C), colocalization between the anti-Myc and streptavidin labels was seen at the bilobe (arrows, area enlarged in insets). In the absence of tetracycline (D), no labeling was observed in either channel. Images are maximum intensity z-projections; overlap was confirmed in single 0.1-μm sections. Identical exposure times and channel level settings were used in image acquisition and processing. Scale bars, 2 μm. (E) Immunoblot of whole-cell lysates using HRP-conjugated streptavidin. In the presence of tetracycline, many additional polypeptides are strongly biotinylated. Equal loading was confirmed by blotting the samples with anti-TbBILBO1 (lower panel).
Fig 3
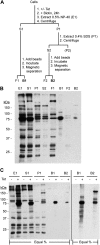
Purification of candidate TbMORN1 binding partners and near neighbors. (A) Schematic of purification protocol. Following incubation in excess biotin and the presence or absence of tetracycline (+/− Tet), the cells are incubated with 0.5% NP-40 (E1) and separated by centrifugation into detergent-soluble (S1) and detergent-insoluble (P1) fractions. The detergent-insoluble fraction is then further extracted with 0.4% SDS. Fractions S1 and S2 are incubated with streptavidin-conjugated paramagnetic beads, and the free unbound fractions (F1 and F2, respectively) are separated magnetically. Fractions B1 and B2 are, respectively, the bound cytoplasmic and cytoskeletal eluates and will contain candidate TbMORN1 binding partners and near neighbors. (B) Representative purification. Myc-BirA*-TbMORN1-expressing cells were processed as described for panel A. Samples were immunoblotted using HRP-conjugated streptavidin. Equal fractions were loaded in each lane. (C) Representative purification from Myc-BirA*-TbMORN1 cells incubated with excess biotin in either the presence or absence of tetracycline. Samples were immunoblotted using HRP-conjugated streptavidin; equal fractions in each lane. Comparison of +/− tetracycline conditions shows clear enrichment of biotinylated proteins in fractions E1, S1, and P1 and purification with minimal background in elutions B1 and B2 (left panel). Loading larger fractions of elutions B1 and B2 shows efficient capture of candidate TbMORN1 binding partners and near neighbors. Background signal from − Tet cells remains minimal (right panel).
Fig 4
Confirmation of expression of tagged candidate proteins. Whole-cell lysates from cells expressing candidate TbMORN1 binding partners and near neighbors were analyzed by immunoblotting with anti-Ty1 antibodies. (A) Western blot of candidates. Expected molecular size is shown next to the figure. Addition of the Ty1 epitope tag causes a distinct decrease in electrophoretic mobility. (B) Dot blot of the two larger candidates.
Fig 5
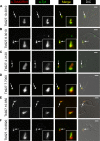
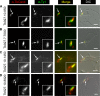
Localization of candidate proteins that overlap TbMORN1. Candidates were expressed with the Ty1 epitope tag in 427 cells either by transient transfection (first five rows) or endogenous replacement (last row). Detergent-extracted cells were labeled with anti-TbMORN1 and anti-Ty1 antibodies and analyzed by fluorescence microscopy. Single 0.1-μm z-slices are shown. Arrows indicate bilobe structure; area enlarged in insets. Fluorescence images were superimposed on differential interference contrast (DIC) images. The candidates localized either to the whole TbMORN1 bilobe (Tb927.10.3010, Tb927.8.3010) (A and B), primarily to the hook posterior part of TbMORN1 (Tb927.4.3120) (C), to both the bilobe and the adjacent TbCentrin4 arm (Tb927.7.7000, Tb927.10.850) (D and E), and to the posterior stem of TbMORN1 (Tb927.10.8820) (F). Scale bars, 2 μm.
Fig 6
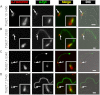
Localization of candidate proteins to the bilobe, FAZ, and flagellum. Candidates were expressed with the Ty1 epitope tag in 427 cells either by endogenous replacement (first two rows) or transient transfection (last two rows). Detergent-extracted cells were labeled with anti-TbMORN1 and anti-Ty1 antibodies and analyzed by fluorescence microscopy. Tb927.10.1450 was localized adjacent to the anterior stem part of TbMORN1 (A). Tb927.7.3330 and Tb927.4.5340 were present on the FAZ and showed partial overlap with TbMORN1 (B and C). Tb927.7.3740 was present on the flagellum (D). Single 0.1-μm z-slices are shown. Arrows indicate bilobe structure; area enlarged in insets. Fluorescence images were superimposed on DIC images. Scale bars, 2 μm.
Fig 7
Localization of candidate TbMORN1 binding partners relative to TbCentrin4. Candidates were expressed with the Ty1 epitope tag in 427 cells either by transient transfection (first two rows) or endogenous replacement (last two rows). Detergent-extracted cells were labeled with anti-TbCentrin4 and anti-Ty1 antibodies and analyzed by fluorescence microscopy. Single 0.1-μm z-slices are shown. Arrows indicate bilobe structure; area enlarged in insets. Fluorescence images were superimposed on DIC images. As expected, Tb927.10.850 and Tb927.7.7000 showed a partial overlap with TbCentrin4 (A and B). Tb927.10.1450 (C) and Tb927.10.8820 (D) showed good and no overlap, respectively. Scale bars, 2 μm.
Fig 8
Schematic summarizing localizations of candidate TbMORN1 binding partners and near neighbors. The image at top left shows a close-up of the bilobe area from a detergent-extracted cell labeled with anti-TbMORN1 and anti-TbCentrin4. Dimensions of the schematic are taken from immunofluorescence images. TbCentrin4 localizations to the basal body and probasal body and the extra density extending from the bar toward the basal body are excluded for clarity.
Similar articles
- Morphology of the trypanosome bilobe, a novel cytoskeletal structure.
Esson HJ, Morriswood B, Yavuz S, Vidilaseris K, Dong G, Warren G. Esson HJ, et al. Eukaryot Cell. 2012 Jun;11(6):761-72. doi: 10.1128/EC.05287-11. Epub 2012 Feb 10. Eukaryot Cell. 2012. PMID: 22327007 Free PMC article. - Proteomic identification of novel cytoskeletal proteins associated with TbPLK, an essential regulator of cell morphogenesis in Trypanosoma brucei.
McAllaster MR, Ikeda KN, Lozano-Núñez A, Anrather D, Unterwurzacher V, Gossenreiter T, Perry JA, Crickley R, Mercadante CJ, Vaughan S, de Graffenried CL. McAllaster MR, et al. Mol Biol Cell. 2015 Sep 1;26(17):3013-29. doi: 10.1091/mbc.E15-04-0219. Epub 2015 Jul 1. Mol Biol Cell. 2015. PMID: 26133384 Free PMC article. - A Novel Basal Body Protein That Is a Polo-like Kinase Substrate Is Required for Basal Body Segregation and Flagellum Adhesion in Trypanosoma brucei.
Hu H, Zhou Q, Li Z. Hu H, et al. J Biol Chem. 2015 Oct 9;290(41):25012-22. doi: 10.1074/jbc.M115.674796. Epub 2015 Aug 13. J Biol Chem. 2015. PMID: 26272611 Free PMC article. - Form, Fabric, and Function of a Flagellum-Associated Cytoskeletal Structure.
Morriswood B. Morriswood B. Cells. 2015 Nov 3;4(4):726-47. doi: 10.3390/cells4040726. Cells. 2015. PMID: 26540076 Free PMC article. Review. - Proteomics and the Trypanosoma brucei cytoskeleton: advances and opportunities.
Portman N, Gull K. Portman N, et al. Parasitology. 2012 Aug;139(9):1168-77. doi: 10.1017/S0031182012000443. Epub 2012 Apr 4. Parasitology. 2012. PMID: 22475638 Review.
Cited by
- Identifying Protein-Protein Associations at the Nuclear Envelope with BioID.
Kim DI, Jensen SC, Roux KJ. Kim DI, et al. Methods Mol Biol. 2016;1411:133-46. doi: 10.1007/978-1-4939-3530-7_8. Methods Mol Biol. 2016. PMID: 27147039 Free PMC article. - An improved smaller biotin ligase for BioID proximity labeling.
Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ. Kim DI, et al. Mol Biol Cell. 2016 Apr 15;27(8):1188-96. doi: 10.1091/mbc.E15-12-0844. Epub 2016 Feb 24. Mol Biol Cell. 2016. PMID: 26912792 Free PMC article. - Structures of three MORN repeat proteins and a re-evaluation of the proposed lipid-binding properties of MORN repeats.
Sajko S, Grishkovskaya I, Kostan J, Graewert M, Setiawan K, Trübestein L, Niedermüller K, Gehin C, Sponga A, Puchinger M, Gavin AC, Leonard TA, Svergun DI, Smith TK, Morriswood B, Djinovic-Carugo K. Sajko S, et al. PLoS One. 2020 Dec 9;15(12):e0242677. doi: 10.1371/journal.pone.0242677. eCollection 2020. PLoS One. 2020. PMID: 33296386 Free PMC article. - The unusual flagellar-targeting mechanism and functions of the trypanosome ortholog of the ciliary GTPase Arl13b.
Zhang Y, Huang Y, Srivathsan A, Lim TK, Lin Q, He CY. Zhang Y, et al. J Cell Sci. 2018 Sep 5;131(17):jcs219071. doi: 10.1242/jcs.219071. J Cell Sci. 2018. PMID: 30097558 Free PMC article. - Paving the Way: Contributions of Big Data to Apicomplexan and Kinetoplastid Research.
Kent RS, Briggs EM, Colon BL, Alvarez C, Silva Pereira S, De Niz M. Kent RS, et al. Front Cell Infect Microbiol. 2022 Jun 6;12:900878. doi: 10.3389/fcimb.2022.900878. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35734575 Free PMC article. Review.
References
- Lacomble S, Vaughan S, Gadelha C, Morphew MK, Shaw MK, McIntosh JR, Gull K. 2009. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J. Cell Sci. 122:1081–1090 doi:<10.1242/jcs.045740>. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials