Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease - PubMed (original) (raw)
. 2013 Jan 8;110(2):636-41.
doi: 10.1073/pnas.1220399110. Epub 2012 Dec 24.
Shannon L Rhodes, Aaron Lulla, Niall P Murphy, Hoa A Lam, Kelley C O'Donnell, Lisa Barnhill, John E Casida, Myles Cockburn, Alvaro Sagasti, Mark C Stahl, Nigel T Maidment, Beate Ritz, Jeff M Bronstein
Affiliations
- PMID: 23267077
- PMCID: PMC3545765
- DOI: 10.1073/pnas.1220399110
Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease
Arthur G Fitzmaurice et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Parkinson disease (PD) is a neurodegenerative disorder particularly characterized by the loss of dopaminergic neurons in the substantia nigra. Pesticide exposure has been associated with PD occurrence, and we previously reported that the fungicide benomyl interferes with several cellular processes potentially relevant to PD pathogenesis. Here we propose that benomyl, via its bioactivated thiocarbamate sulfoxide metabolite, inhibits aldehyde dehydrogenase (ALDH), leading to accumulation of the reactive dopamine metabolite 3,4-dihydroxyphenylacetaldehyde (DOPAL), preferential degeneration of dopaminergic neurons, and development of PD. This hypothesis is supported by multiple lines of evidence. (i) We previously showed in mice the metabolism of benomyl to S-methyl N-butylthiocarbamate sulfoxide, which inhibits ALDH at nanomolar levels. We report here that benomyl exposure in primary mesencephalic neurons (ii) inhibits ALDH and (iii) alters dopamine homeostasis. It induces selective dopaminergic neuronal damage (iv) in vitro in primary mesencephalic cultures and (v) in vivo in a zebrafish system. (vi) In vitro cell loss was attenuated by reducing DOPAL formation. (vii) In our epidemiology study, higher exposure to benomyl was associated with increased PD risk. This ALDH model for PD etiology may help explain the selective vulnerability of dopaminergic neurons in PD and provide a potential mechanism through which environmental toxicants contribute to PD pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
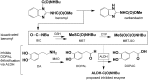
Fig. 1.
ALDH inhibition as the proposed mechanism of benomyl-induced Parkinson disease. Benomyl is efficiently metabolized to potent ALDH inhibitors—BIC, MBT, and particularly MBT-SO—so exposure leads to the accumulation of the toxic dopamine metabolite DOPAL. This offers a possible explanation for the selective toxicity to dopaminergic neurons observed in PD pathogenesis. GSH, glutathione.
Fig. 2.
Inhibitory actions of benomyl and its metabolites. (A) Exposure to benomyl or MBT, but not carbendazim, inhibited ex vivo ALDH activity in mesencephalic neurons dissociated from 2-d-old rat pups (n = 3–11). (B) Benomyl, BIC, and MBT inhibited in vitro mitochondrial ALDH activity (n = 2–8 at each concentration tested). Carbendazim did not significantly inhibit mALDH activity at up to 20 μM (n = 4). (C) Benomyl and carbendazim inhibited 26S UPS activity in SK-_N_-MC neuroblastoma cells (n = 4–14 at each concentration tested), whereas MBT exposure up to 10 μM had no effect (n = 5). (D) Benomyl/BIC/MBT inhibit ALDH activity at lower concentrations than benomyl/carbendazim inhibit the 26S UPS. Data are expressed as percent relative to vehicle controls (0.01% DMSO), except for 26S UPS inhibition which is relative to treatment with the 26S UPS inhibitor lactacystin (5 μM). *P < 0.01, **P < 0.0001; benomyl (○), BIC (◇), MBT (☐), carbendazim (Δ). mALDH, mitochondrial aldehyde dehydrogenase; 26S UPS, ubiquitin-proteasome system.
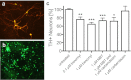
Fig. 3.
Dopaminergic neuronal damage in primary mesencephalic cultures exposed to benomyl or its metabolites. Representative field of view (20×) shows untreated immunoreactive (A) dopaminergic neurons (TH+) and (B) neuronal nuclei (NeuN+). (C) Benomyl neurotoxicity was recapitulated by MBT exposure, whereas carbendazim was not toxic to TH+ neurons at 1 μM. NeuN+ counts were unaffected by any treatment. Because MBT is either the proximal or penultimate benomyl metabolite that inhibits ALDH activity, there appears to be an association between neuronal damage and ALDH inhibition; proteasomal inhibition by the carbendazim moiety is not sufficient to kill cells under the same conditions. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P < 0.05, **P < 0.01, ***P < 0.0001.
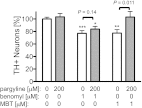
Fig. 4.
Monoamine oxidase (MAO) inhibitor protects against neurotoxicity because of DOPAL accumulation. Neuronal loss resulting from 1 μM benomyl or MBT exposure was mitigated by cotreatment with the MAO inhibitor pargyline (200 μM, n = 13–28). Because MAO inhibition reduces the metabolism of dopamine to DOPAL, this suggests that DOPAL is toxic to dopaminergic neurons and that benomyl is toxic via DOPAL accumulation as a result of ALDH inhibition. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P = 0.0027, **P = 2.4 × 10−4, ***P = 6.1 × 10−5.
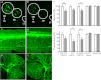
Fig. 5.
Aminergic neuronal damage in Danio rerio embryos exposed to benomyl. Representative confocal images of zebrafish embryos (A, C, and E) unexposed or (B, D, and F) bathed in 1 μM benomyl from 5 h until 120 h postfertilization are shown. (G) Neuronal counts (A and B) decreased in VMAT2+ anterior and diencephalic clusters of ETvmat2:GFP zebrafish exposed to benomyl (solid bars) but were unaffected in (C and D) Rohon-Beard and (E and F) trigeminal neurons in Tg(isl1[ss]:Gal4-VP16,UAS:EGFP) zf154 zebrafish. (H) Measurement of total fluorescence yielded similar results. Data are expressed as percent relative to vehicle controls (0.01% DMSO). *P < 0.1, **P < 0.05. De, diencephalon; LC, locus coeruleus; OB, olfactory bulb; Te, telencephalon.
Similar articles
- Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson's disease.
Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS. Casida JE, et al. Chem Res Toxicol. 2014 Aug 18;27(8):1359-61. doi: 10.1021/tx5002223. Epub 2014 Jul 24. Chem Res Toxicol. 2014. PMID: 25045800 Free PMC article. - Rotenone decreases intracellular aldehyde dehydrogenase activity: implications for the pathogenesis of Parkinson's disease.
Goldstein DS, Sullivan P, Cooney A, Jinsmaa Y, Kopin IJ, Sharabi Y. Goldstein DS, et al. J Neurochem. 2015 Apr;133(1):14-25. doi: 10.1111/jnc.13042. Epub 2015 Feb 25. J Neurochem. 2015. PMID: 25645689 Free PMC article. - Mechanism for benomyl action as a mitochondrial aldehyde dehydrogenase inhibitor in mice.
Staub RE, Quistad GB, Casida JE. Staub RE, et al. Chem Res Toxicol. 1998 May;11(5):535-43. doi: 10.1021/tx980002l. Chem Res Toxicol. 1998. PMID: 9585485 - Aldehyde dehydrogenase inhibition generates a reactive dopamine metabolite autotoxic to dopamine neurons.
Doorn JA, Florang VR, Schamp JH, Vanle BC. Doorn JA, et al. Parkinsonism Relat Disord. 2014 Jan;20 Suppl 1(0 1):S73-5. doi: 10.1016/S1353-8020(13)70019-1. Parkinsonism Relat Disord. 2014. PMID: 24262193 Free PMC article. Review. - 3,4-dihydroxyphenylacetaldehyde: a potential target for neuroprotective therapy in Parkinson's disease.
Burke WJ. Burke WJ. Curr Drug Targets CNS Neurol Disord. 2003 Apr;2(2):143-8. doi: 10.2174/1568007033482913. Curr Drug Targets CNS Neurol Disord. 2003. PMID: 12769806 Review.
Cited by
- Role and Mechanism of Vitamin A Metabolism in the Pathophysiology of Parkinson's Disease.
Marie A, Darricau M, Touyarot K, Parr-Brownlie LC, Bosch-Bouju C. Marie A, et al. J Parkinsons Dis. 2021;11(3):949-970. doi: 10.3233/JPD-212671. J Parkinsons Dis. 2021. PMID: 34120916 Free PMC article. Review. - DOPAL initiates αSynuclein-dependent impaired proteostasis and degeneration of neuronal projections in Parkinson's disease.
Masato A, Plotegher N, Terrin F, Sandre M, Faustini G, Thor A, Adams S, Berti G, Cogo S, De Lazzari F, Fontana CM, Martinez PA, Strong R, Bandopadhyay R, Bisaglia M, Bellucci A, Greggio E, Dalla Valle L, Boassa D, Bubacco L. Masato A, et al. NPJ Parkinsons Dis. 2023 Mar 25;9(1):42. doi: 10.1038/s41531-023-00485-1. NPJ Parkinsons Dis. 2023. PMID: 36966140 Free PMC article. - Alterations in the nigrostriatal dopamine system after acute systemic PhIP exposure.
Agim ZS, Cannon JR. Agim ZS, et al. Toxicol Lett. 2018 May 1;287:31-41. doi: 10.1016/j.toxlet.2018.01.017. Epub 2018 Jan 31. Toxicol Lett. 2018. PMID: 29378243 Free PMC article. - Advances in Enzyme-Based Biosensors for Pesticide Detection.
Bucur B, Munteanu FD, Marty JL, Vasilescu A. Bucur B, et al. Biosensors (Basel). 2018 Mar 22;8(2):27. doi: 10.3390/bios8020027. Biosensors (Basel). 2018. PMID: 29565810 Free PMC article. Review. - Biogenic Aldehyde-Mediated Mechanisms of Toxicity in Neurodegenerative Disease.
Cagle BS, Crawford RA, Doorn JA. Cagle BS, et al. Curr Opin Toxicol. 2019 Feb;13:16-21. doi: 10.1016/j.cotox.2018.12.002. Epub 2018 Dec 17. Curr Opin Toxicol. 2019. PMID: 31304429 Free PMC article.
References
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. - PubMed
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: A metaanalysis. Environ Res. 2001;86(2):122–127. - PubMed
- Ascherio A, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60(2):197–203. - PubMed
- Firestone JA, et al. Pesticides and risk of Parkinson disease: A population-based case-control study. Arch Neurol. 2005;62(1):91–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 ES012078/ES/NIEHS NIH HHS/United States
- T32 GM008042/GM/NIGMS NIH HHS/United States
- P01ES016732/ES/NIEHS NIH HHS/United States
- P01 ES016732/ES/NIEHS NIH HHS/United States
- U54ES012078/ES/NIEHS NIH HHS/United States
- T32ES015457/ES/NIEHS NIH HHS/United States
- T32 ES015457/ES/NIEHS NIH HHS/United States
- NS038367/NS/NINDS NIH HHS/United States
- R01ES010544/ES/NIEHS NIH HHS/United States
- R21 ES016446/ES/NIEHS NIH HHS/United States
- R01 ES010544/ES/NIEHS NIH HHS/United States
- P50 NS038367/NS/NINDS NIH HHS/United States
- 5R21ES16446-2/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases