Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death - PubMed (original) (raw)
Subversion of autophagy in adherent invasive Escherichia coli-infected neutrophils induces inflammation and cell death
Abderrahman Chargui et al. PLoS One. 2012.
Abstract
Invading bacteria are recognized, captured and killed by a specialized form of autophagy, called xenophagy. Recently, defects in xenophagy in Crohn's disease (CD) have been implicated in the pathogenesis of human chronic inflammatory diseases of uncertain etiology of the gastrointestinal tract. We show here that pathogenic adherent-invasive Escherichia coli (AIEC) isolated from CD patients are able to adhere and invade neutrophils, which represent the first line of defense against bacteria. Of particular interest, AIEC infection of neutrophil-like PLB-985 cells blocked autophagy at the autolysosomal step, which allowed intracellular survival of bacteria and exacerbated interleukin-8 (IL-8) production. Interestingly, this block in autophagy correlated with the induction of autophagic cell death. Likewise, stimulation of autophagy by nutrient starvation or rapamycin treatment reduced intracellular AIEC survival and IL-8 production. Finally, treatment with an inhibitor of autophagy decreased cell death of AIEC-infected neutrophil-like PLB-985 cells. In conclusion, excessive autophagy in AIEC infection triggered cell death of neutrophils.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
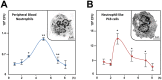
Figure 1. AIEC invade and replicate within PMN.
A) Peripheral blood PMNs or B) differentiated PLB-985 cells were infected with AIEC LF82 at a multiplicity of infection (MOI) of 50. The survival of bacteria was measured by the gentamicin protection assay. After 1 h of infection (50 MOI), peripheral blood PMNs and differentiated PLB-985 cells were incubated with gentamicin (100 µg/ml). At the indicated times, cells were washed with PBS and lysed with PBS 1% Triton X-100. The CFUs were determined on LB agar plates. The data are representative of 5 independent experiments. Inset: representative transmission electron micrograph (TEM) pictures of differentiated PLB-985 cells showing the characteristic segmented nuclei of mature PMN with connected lobes. * p<0.05, ** p<0.02.
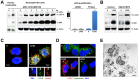
Figure 2. Inhibition of autophagic flux by AIEC LF82 infection.
Human neutrophils or neutrophil-like PLB-985 cells were infected with AIEC LF82 at a MOI of 50 for 1 h than gentamicin (100 µg/ml) was added for 3 h. Cells were and processed for immunoblotting (A, left panel, B), quantitative RT-PCR (A rigth panel), immunofluorescence (C, D) and ultrastructural TEM analysis (E). (A) Time-dependent accumulation (1–8 h) of LC3-II and p62 in infected human neutrophils or neutrophil-like PLB-985 cells compared to uninfected cells analyzed at the end time point. Longer exposure detects the LC3-I band. We checked that AIEC infection did not affect p62 mRNA levels by qRT-PCR analysis (A right panel). Data are means ± SEM of three experiments. ** p<0.001. (B) Autophagic flux was analysed by immunoblot analysis in differentiated PLB-985 cells infected for 3 h with AIEC LF82 bacteria (MOI 50) in the absence or in the presence of E64d/PEPS. Actin was used as a loading control. Control unstimulated cells were analysed at the end time point. (C) Representative confocal images of control (0) or infected cells (LF82) (3 h post infection) showed the colocalization of bacteria with LC3-II and LAMP-1 proteins as indicated by yellow punctiform staining. Insets highlight individual staining of bacteria (DNA staining, blue), LC3-II (Alexa 488, green) and LAMP-1 (Alexa 594, red). (D) Representative confocal micrographs of control (0) and LF82 infected cells (LF82) showing the co-localization of bacteria (DNA staining, blue) within autophagic (LC3-II positive, Alexa 594, red) but not acidic compartments (LysoTracker negative, green). (E) Representative TEM images showing bacteria within endosomes (asterisk), autophagosomes (arrowheads) or free in the cytosol (arrow) in LF82-infected cells (3 h post infection). Bar = 2 µm.
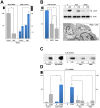
Figure 3. Modulation of autophagy affects AIEC survival.
PLB-985 cells were transduced with either Atg5 shRNA or the control shRNA, differentiated and then infected with LF82 (50 MOI). (A) A defect in autophagy (Atg5 silencing) favours intracellular survival of bacteria. Intracellular bacteria were numbered 1 h and 5 h after addition of gentamycin. Data are means ± SEM of three experiments. *<0.01 and **p<0.003. Silencing of Atg5 mRNA was confirmed by RT-PCR analysis (left). (B) Two inducers of autophagy, nutrient starvation (HBSS) and rapamycin (rapa, 100 nM in complete medium) decreased intracellular LF82 survival (left), rescued the autophagic flux (as shown by detection of LC3-II, upper right), and increased bacterial degradation (lower right, arrowhead, transmission electron micrograph). Modulation of LF82-induced IL-8 production in response to inhibition of autophagy (Atg5 shRNA) or to stimulation of autophagy (rapamycin or starvation) was analyzed by immunoblotting (C) and qRT-PCR (D). The average ± S.D. is shown for three independent experiments, * <0.01 and ** p<0.003.
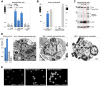
Figure 4. PMNs undergo autophagic death and NETosis on infection with AIEC LF82.
Differentiated PLB-985 cells or PMN were infected (MOI 50) with K12 or AIEC LF82 bacteria for 1 h and gentamycin was added for the following 5 h, cells were then analysed. (A) Control or K12- or AIEC LF82-infected PLB-985 cells, treated or not with 3-methyladenine (3-MA, 5 mM, autophagy inhibitor) or Zvad (5 µM, a pancaspase inhibitor), were incubated with propidium iodide (PI) (5 µg/ml) and cell death was analysed by flow cytometric analysis as described in the materials and methods section. The average ± S.D. is shown for three independent experiments, *p<0.003. Inset: immunoblot analysis of LC3-II testifying to the induction of autophagy in infected cells. (B, left panel) PMNs were infected with AIEC LF82 and cell death was assayed as described in A. Maximal cell death (+) was obtained after treatment with etoposide phosphate (100 µg/ml). To test whether cell death was due to apoptosis, a caspase-3 activity test was performed as described in the materials and methods section (right panel). Maximal cell apoptosis (+) was obtained by treatment with staurosporine (10 µM). (C) Cleavage of PARP and caspase-3 in non-infected or K12- and AIEC LF82-infected differentiated PLB-985 cells were analysed by immunoblot analyses. Etoposide phosphate (100 µg/ml) was used as a positive control. LC3-II was detected in LF82-infected cells and compared to uninfected or K12-infected cells (1+5 h, MOI 50). Actin was used as a loading control. (D) Survival of AIEC LF82 and K12 in differentiated PLB-985 cells was analysed with the gentamycin assay (panel one). To compare the ultrastructural morphology of vesicles and bacteria in PLB-985 cells transmission electron microscopy was performed. Ultrastructural analysis of K12-infected differentiated PLB-985 cells shows bacterial sequestration and degradation in phagocytosis vacuoles (panel two). In contrast, accumulation of autophagic vesicles and bacteria (arrowheads) inside the vacuoles was observed in PLB-985 cells (panel three) and PMNs (panel four). Scale bar = 1 µm. (E) Differentiated PLB-985 were seeded on glass coverslips coated with poly D lysine (5 µg/cm2) and allowed to settle for 1 h. Cells were then infected with AIEC-LF82 (MOI 50) for 4 h (1 h+3 h) and 6 h (1 h+5 h), fixed with 3% paraformaldehyde and stained with Hoechst 33342 (0.5 µg/ml) to visualize DNA. Cells were examined with an epifluorescence Axiophot microscope (Zeiss). Early apoptosis and necrosis were assayed by measuring Annexin-V-fluos (Roche) and propidium iodide (PI). Differentiated non-infected PLB-985 cells or cells infected with AIEC LF82 (MOI 50) for 1 h+5 h were incubated with Annexin-V-fluos and PI and fluorescence was detected on a FACS Calibure.
Similar articles
- Defects in autophagy favour adherent-invasive Escherichia coli persistence within macrophages leading to increased pro-inflammatory response.
Lapaquette P, Bringer MA, Darfeuille-Michaud A. Lapaquette P, et al. Cell Microbiol. 2012 Jun;14(6):791-807. doi: 10.1111/j.1462-5822.2012.01768.x. Epub 2012 Mar 1. Cell Microbiol. 2012. PMID: 22309232 - HIF1A regulates xenophagic degradation of adherent and invasive Escherichia coli (AIEC).
Mimouna S, Bazin M, Mograbi B, Darfeuille-Michaud A, Brest P, Hofman P, Vouret-Craviari V. Mimouna S, et al. Autophagy. 2014;10(12):2333-45. doi: 10.4161/15548627.2014.984275. Autophagy. 2014. PMID: 25484075 Free PMC article. - Crohn's disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy.
Nguyen HT, Dalmasso G, Müller S, Carrière J, Seibold F, Darfeuille-Michaud A. Nguyen HT, et al. Gastroenterology. 2014 Feb;146(2):508-19. doi: 10.1053/j.gastro.2013.10.021. Epub 2013 Oct 19. Gastroenterology. 2014. PMID: 24148619 - Pathogenesis of adherent-invasive Escherichia coli.
Smith EJ, Thompson AP, O'Driscoll A, Clarke DJ. Smith EJ, et al. Future Microbiol. 2013 Oct;8(10):1289-300. doi: 10.2217/fmb.13.94. Future Microbiol. 2013. PMID: 24059919 Review. - Adherent-invasive Escherichia coli in inflammatory bowel disease.
Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, Barnich N, Ng SC, Colombel JF. Palmela C, et al. Gut. 2018 Mar;67(3):574-587. doi: 10.1136/gutjnl-2017-314903. Epub 2017 Nov 15. Gut. 2018. PMID: 29141957 Review.
Cited by
- The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans.
Stoiber W, Obermayer A, Steinbacher P, Krautgartner WD. Stoiber W, et al. Biomolecules. 2015 May 4;5(2):702-23. doi: 10.3390/biom5020702. Biomolecules. 2015. PMID: 25946076 Free PMC article. Review. - Neutrophils: Many Ways to Die.
Pérez-Figueroa E, Álvarez-Carrasco P, Ortega E, Maldonado-Bernal C. Pérez-Figueroa E, et al. Front Immunol. 2021 Mar 4;12:631821. doi: 10.3389/fimmu.2021.631821. eCollection 2021. Front Immunol. 2021. PMID: 33746968 Free PMC article. Review. - Incomplete autophagy promotes the proliferation of Mycoplasma hyopneumoniae through the JNK and Akt pathways in porcine alveolar macrophages.
Wen Y, Chen Z, Tian Y, Yang M, Dong Q, Yang Y, Ding H. Wen Y, et al. Vet Res. 2022 Aug 4;53(1):62. doi: 10.1186/s13567-022-01074-5. Vet Res. 2022. PMID: 35927699 Free PMC article. - Making the Most of the Host; Targeting the Autophagy Pathway Facilitates Staphylococcus aureus Intracellular Survival in Neutrophils.
Vozza EG, Mulcahy ME, McLoughlin RM. Vozza EG, et al. Front Immunol. 2021 Jun 16;12:667387. doi: 10.3389/fimmu.2021.667387. eCollection 2021. Front Immunol. 2021. PMID: 34220813 Free PMC article. Review. - Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity.
Martinez-Medina M, Garcia-Gil LJ. Martinez-Medina M, et al. World J Gastrointest Pathophysiol. 2014 Aug 15;5(3):213-27. doi: 10.4291/wjgp.v5.i3.213. World J Gastrointest Pathophysiol. 2014. PMID: 25133024 Free PMC article. Review.
References
- Mimouna S, Goncalves D, Barnich N, Darfeuille-Michaud A, Hofman P, et al. (2011) Crohn disease-associated Escherichia coli promote gastrointestinal inflammatory disorders by activation of HIF-dependent responses. Gut Microbes 2: 335–346. - PubMed
- Cesaro A, Brest P, Hofman V, Hebuterne X, Wildman S, et al. (2010) Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am J Physiol Gastrointest Liver Physiol 299: G32–42. - PubMed
- von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, et al. (2005) Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106: 1423–1431. - PubMed
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. - PubMed
- Mihalache CC, Yousefi S, Conus S, Villiger PM, Schneider EM, et al. (2011) Inflammation-associated autophagy-related programmed necrotic death of human neutrophils characterized by organelle fusion events. J Immunol 186: 6532–6542. - PubMed
Publication types
MeSH terms
Grants and funding
Funding provided by Infectiopôle Sud PACA (A. Chargui: postdoctoral fellowship), Association François Aupetit, “Institut National de la Santé et de la Recherche Médicale”, “Agence de l’Environnement et de la Maîtrise de l’Energie” (A B and A. Cesaro: convention ADEME n° 08 62 C 0044), “Agence régionale santé Provence Alpes Côte d’Azur and Direction régionale de l’Environnement, de l’aménagement et du logement” (A B: plan régional santé environnement PRSE PACA n°6.3.3.3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources