Aquaporin-4-dependent K(+) and water transport modeled in brain extracellular space following neuroexcitation - PubMed (original) (raw)
Aquaporin-4-dependent K(+) and water transport modeled in brain extracellular space following neuroexcitation
Byung-Ju Jin et al. J Gen Physiol. 2013 Jan.
Abstract
Potassium (K(+)) ions released into brain extracellular space (ECS) during neuroexcitation are efficiently taken up by astrocytes. Deletion of astrocyte water channel aquaporin-4 (AQP4) in mice alters neuroexcitation by reducing ECS [K(+)] accumulation and slowing K(+) reuptake. These effects could involve AQP4-dependent: (a) K(+) permeability, (b) resting ECS volume, (c) ECS contraction during K(+) reuptake, and (d) diffusion-limited water/K(+) transport coupling. To investigate the role of these mechanisms, we compared experimental data to predictions of a model of K(+) and water uptake into astrocytes after neuronal release of K(+) into the ECS. The model computed the kinetics of ECS [K(+)] and volume, with input parameters including initial ECS volume, astrocyte K(+) conductance and water permeability, and diffusion in astrocyte cytoplasm. Numerical methods were developed to compute transport and diffusion for a nonstationary astrocyte-ECS interface. The modeling showed that mechanisms b-d, together, can predict experimentally observed impairment in K(+) reuptake from the ECS in AQP4 deficiency, as well as altered K(+) accumulation in the ECS after neuroexcitation, provided that astrocyte water permeability is sufficiently reduced in AQP4 deficiency and that solute diffusion in astrocyte cytoplasm is sufficiently low. The modeling thus provides a potential explanation for AQP4-dependent K(+)/water coupling in the ECS without requiring AQP4-dependent astrocyte K(+) permeability. Our model links the physical and ion/water transport properties of brain cells with the dynamics of neuroexcitation, and supports the conclusion that reduced AQP4-dependent water transport is responsible for defective neuroexcitation in AQP4 deficiency.
Figures
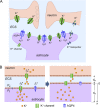
Figure 1.
Model of K+ and water transport in brain ECS. (A) Diagram showing neurons and astrocytes in brain surrounded by an ECS. (B) Schematic of mathematical model. Neuronal, ECS, and astrocytic compartments are shown, along with key transport mechanisms including neuronal K+ release (JKn), K+ uptake by astrocytes (JKa), and osmotic water transport into astrocytes (JVa).
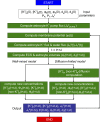
Figure 2.
Computational approach. See the Model computations section for description.
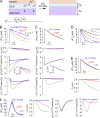
Figure 3.
Predictions of the well-mixed model. (A) Schematic of well-mixed model showing neuronal K+ release into ECS, and astrocytic uptake of K+ and water causing ECS shrinkage. (B) Time course of ECS potassium concentration ([K+]e) and volume (de), astrocyte membrane potential (ψ), and astrocyte K+ and water flux (JKa, JVa) after neuronal excitation causing [K+]e increase from 5 to 10 mM. Parameters: Pf = 0.04 cm/s, JKno = 10−8 mol/cm2/s, Δtn = 0.1 s, da = 10 µm, de = 2 µm, with the indicated PK. (C) Computations as in B for PK = 1.2 × 10−5 cm/s with the indicated Pf. (D) Computations as in B for PK = 1.2 × 10−5 cm/s, with the indicated de. JKno was adjusted to increase [K+]e from 5 to 10 mM. (E) Effect of magnitude of neuroexcitation. Computations as in B, with PK = 1.5 × 10−5 cm/s and JKno = 5 × 10−8 mol/cm2/s, with the indicated Pf. (F) The effect of duration of neuroexcitation. Computations as in B, with PK = 1.3 × 10−5 cm/s, JKno = 8 × 10−10 mol/cm2/s, and Δtn = 10 s, with the indicated Pf.
Figure 4.
Predictions of the diffusion-limited model for high astrocyte water permeability. Pf = 0.04 cm/s for computations in this figure. (A) Schematic showing nonlinear and newly added mesh elements in astrocyte cytoplasm used for numerical solution of the diffusion equation. See text for explanation. (B) Time course of [K+]e, de, ψ, JKa, and JVa after neuroexcitation for the indicated cytoplasmic diffusion coefficients, Da. Parameters: PK = 1.2 × 10−5 cm/s, JKno = 10−8 mol/cm2/s, Δtn = 0.1 s, da = 10 µm, and de = 2 µm. (C and D) Astrocyte spatial distribution of [K+]a and [non-K+]a at the indicated times, with parameters as in B, for Da = 10−8 cm2/s (C) and t = 5 s (D). (E) Effect of duration of neuroexcitation. Computations as in B, with PK = 1.3 × 10−5 cm/s, JKno = 8 × 10−10 mol/cm2/s, and Δtn = 10 s.
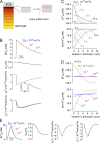
Figure 5.
Water permeability effects in the diffusion-limited model. (A) Time course of [K+]e and de for the indicated Pf and Da. Parameters: PK = 1.2 × 10−5 cm/s, JKno = 10−8 mol/cm2/s, Δtn = 0.1 s, da = 10 µm, and de = 2 µm. (B) Effect of magnitude of neuroexcitation. Computations as in B, but with JKno = 5 × 10−8 mol/cm2/s to increase [K+]e from 5 to 30 mM. (C) Effect of duration of neuroexcitation. Computations as in C, with PK = 1.3 × 10−5 cm/s, JKno = 8 × 10−10 mol/cm2/s, and Δtn = 10 s. (D) Spatial distributions of [K+]a and osmolarity (Φa) in astrocyte cytoplasm for the indicated Pf and Da, with parameters as in C.
Figure 6.
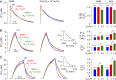
Modeling of experimental observations of altered K+ dynamics in AQP4 deficiency. (A) Single neuron firing (Δtn = 0.1 ms). Time course of [K+]e modeled for wild type (WT) mice (Pf = 0.04 cm/s) and AQP4 knockout mice (AQP4−/−, Pf = 0.001 cm/s, de = 2 or 2.4 µm) for well-mixed model (WMM, left) and diffusion-limited model (DLM, Da = 10−9 cm2/s, center). Parameters: PKa = 1.2 × 10−5 cm/s, JKno = 3.5 × 10−7 mol/cm2/s, and Δtn = 0.1 ms. (right) Relative increase [K+]e after neuroexcitation in WT vs. AQP4 knockout mice (Δ[K+]e−/−/Δ[K+]e+/+). (B) Repetitive pulsed neuronal excitation. (left and center) [K+]e in response to 20 Hz stimulation (Δtn = 0.1 ms firing) for 10 s. Parameters: PKa = 1.2 × 10−5 cm/s and JKno = 2.1 × 10−7 mol/cm2/s. (right) [K+]e at 20 s (broken line) after neuroexcitation and relative half-times for return of [K+]e to baseline (t1/2−/−/t1/2+/+). (inset) Pf dependence of t1/2 in the DLM (at fixed de of 2 µm), shown on a normalized y scale (denoted <t1/2>). A similar Pf dependence was seen for [K+]e (not depicted). (C) Prolonged neuronal firing. (left and center) [K+]e responses to 20 Hz stimulation for 30 s. Parameters: PKa = 1.2 × 10−5 cm/s and JKno = 3.45 × 10−7 mol/cm2/s. (right) Summary of t1/2−/−/t1/2+/+. (inset) Pf dependence of <t1/2> in the DLM (at fixed de of 2 µm), shown on a normalized y scale.
Similar articles
- The impact of alpha-syntrophin deletion on the changes in tissue structure and extracellular diffusion associated with cell swelling under physiological and pathological conditions.
Dmytrenko L, Cicanic M, Anderova M, Vorisek I, Ottersen OP, Sykova E, Vargova L. Dmytrenko L, et al. PLoS One. 2013 Jul 5;8(7):e68044. doi: 10.1371/journal.pone.0068044. Print 2013. PLoS One. 2013. PMID: 23861848 Free PMC article. - Potassium dependent regulation of astrocyte water permeability is mediated by cAMP signaling.
Song Y, Gunnarson E. Song Y, et al. PLoS One. 2012;7(4):e34936. doi: 10.1371/journal.pone.0034936. Epub 2012 Apr 6. PLoS One. 2012. PMID: 22493723 Free PMC article. - Aquaporin-4 regulates the velocity and frequency of cortical spreading depression in mice.
Yao X, Smith AJ, Jin BJ, Zador Z, Manley GT, Verkman AS. Yao X, et al. Glia. 2015 Oct;63(10):1860-9. doi: 10.1002/glia.22853. Epub 2015 May 6. Glia. 2015. PMID: 25944186 Free PMC article. - Regulation and Function of AQP4 in the Central Nervous System.
Assentoft M, Larsen BR, MacAulay N. Assentoft M, et al. Neurochem Res. 2015 Dec;40(12):2615-27. doi: 10.1007/s11064-015-1519-z. Epub 2015 Jan 29. Neurochem Res. 2015. PMID: 25630715 Review. - Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes.
Jorgačevski J, Zorec R, Potokar M. Jorgačevski J, et al. Cells. 2020 Dec 7;9(12):2622. doi: 10.3390/cells9122622. Cells. 2020. PMID: 33297299 Free PMC article. Review.
Cited by
- Mechanisms Underlying Aquaporin-4 Subcellular Mislocalization in Epilepsy.
Szu JI, Binder DK. Szu JI, et al. Front Cell Neurosci. 2022 Jun 6;16:900588. doi: 10.3389/fncel.2022.900588. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35734218 Free PMC article. - Involvement of aquaporin 4 in astrocyte function and neuropsychiatric disorders.
Xiao M, Hu G. Xiao M, et al. CNS Neurosci Ther. 2014 May;20(5):385-90. doi: 10.1111/cns.12267. Epub 2014 Apr 8. CNS Neurosci Ther. 2014. PMID: 24712483 Free PMC article. Review. - Aquaporins: important but elusive drug targets.
Verkman AS, Anderson MO, Papadopoulos MC. Verkman AS, et al. Nat Rev Drug Discov. 2014 Apr;13(4):259-77. doi: 10.1038/nrd4226. Epub 2014 Mar 14. Nat Rev Drug Discov. 2014. PMID: 24625825 Free PMC article. Review. - Response to "When can AQP4 assist transporter-mediated K⁺ uptake?".
Jin BJ, Zhang H, Binder DK, Verkman AS. Jin BJ, et al. J Gen Physiol. 2013 Jul;142(1):91-2. doi: 10.1085/jgp.201311010. Epub 2013 Jun 10. J Gen Physiol. 2013. PMID: 23752331 Free PMC article. No abstract available. - Diffusion in the extracellular space in brain and tumors.
Verkman AS. Verkman AS. Phys Biol. 2013 Aug;10(4):045003. doi: 10.1088/1478-3975/10/4/045003. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23913007 Free PMC article.
References
- Amiry-Moghaddam M., Williamson A., Palomba M., Eid T., de Lanerolle N.C., Nagelhus E.A., Adams M.E., Froehner S.C., Agre P., Ottersen O.P. 2003. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc. Natl. Acad. Sci. USA. 100:13615–13620 10.1073/pnas.2336064100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical