Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing - PubMed (original) (raw)
. 2013 Jan 2;33(1):315-26.
doi: 10.1523/JNEUROSCI.2804-12.2013.
Katrin Kistner, Michelino Puopolo, David Segal, David Roberson, Marco Sisignano, Sandra Labocha, Nerea Ferreirós, Amanda Strominger, Enrique J Cobos, Nader Ghasemlou, Gerd Geisslinger, Peter W Reeh, Bruce P Bean, Clifford J Woolf
Affiliations
- PMID: 23283344
- PMCID: PMC3640269
- DOI: 10.1523/JNEUROSCI.2804-12.2013
Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing
Christian Brenneis et al. J Neurosci. 2013.
Abstract
Specific somatosensations may be processed by different subsets of primary afferents. C-fibers expressing heat-sensitive TRPV1 channels are proposed, for example, to be heat but not mechanical pain detectors. To phenotype in rats the sensory function of TRPV1(+) afferents, we rapidly and selectively silenced only their activity, by introducing the membrane-impermeant sodium channel blocker QX-314 into these axons via the TRPV1 channel pore. Using tandem mass spectrometry we show that upon activation with capsaicin, QX-314 selectively accumulates in the cytosol only of TRPV1-expressing cells, and not in control cells. Exposure to QX-314 and capsaicin induces in small DRG neurons a robust sodium current block within 30 s. In sciatic nerves, application of extracellular QX-314 with capsaicin persistently reduces C-fiber but not A-fiber compound action potentials and this effect does not occur in TRPV1(-/-) mice. Behavioral phenotyping after selectively silencing TRPV1(+) sciatic nerve axons by perineural injections of QX-314 and capsaicin reveals deficits in heat and mechanical pressure but not pinprick or light touch perception. The response to intraplantar capsaicin is substantially reduced, as expected. During inflammation, silencing TRPV1(+) axons abolishes heat, mechanical, and cold hyperalgesia but tactile and cold allodynia remain following peripheral nerve injury. These results indicate that TRPV1-expressing sensory neurons process particular thermal and mechanical somatosensations, and that the sensory channels activated by mechanical and cold stimuli to produce pain in naive/inflamed rats differ from those in animals after peripheral nerve injury.
Figures
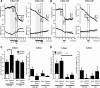
Figure 1.
QX-314 accumulates selectively in TRPV1-expressing cells upon capsaicin stimulation. A, Concentration–response curve for TRPV1-dependent QX-314 accumulation. CHO cells were stably transfected with control (pcDNA3.1) or rTRPV1 expression plasmids and incubated with capsaicin (1 μ
m
) and the indicated extracellular QX-314 concentrations for 10 min. QX-314 was determined from cell lysates by LC–MS/MS. Each data point represents a mean of six culture dishes. B, TRPV1-dependent QX-314 accumulation in the cytosolic fraction. HEK-293 cells were stably transfected with a doxycyclin-inducible hTRPV1 plasmid and induced 15 h before incubation with 500 μ
m
QX-314 ± 1 μ
m
capsaicin for 10 min. QX-314 concentrations were determined from the cytosolic fractions after ultracentrifugation by LC–MS/MS (n = 3). C, Same conditions as in B but membrane fractions were analyzed for QX-314 concentration (n = 3). D, Time course for the release of QX-314 from cells incubated as in B and C. After incubating QX-314-loaded TRPV1-expressing cells in fresh medium for different durations, QX-314 was determined in the cell culture medium (1 ml) or in cell lysates (300 μ
m
) (n = 3). Data represent the average of the absolute drug amount in both solutions ± SEM. Significance was calculated by two-way ANOVA and Bonferroni correction. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells expressing no TRPV1.
Figure 2.
Time course of sodium channel inhibition in DRG neurons by QX-314 following entry through TRPV1 channels and by internal application of QX-314. A, Time course of sodium channel inhibition by QX-314 and capsaicin applied externally to voltage-clamped rat DRG neurons. Sodium current was elicited by a voltage step from −80 to 0 mV delivered every second. Ten millimole QX-314 and 1 μ
m
capsaicin were coapplied for multiple 10 s periods, each exposure period separated by 40 s of washing by control solution to reverse the capsaicin-activated TRPV1 current. B, Combined results for the experiment in A performed in five DRG neurons (solid circles). In addition, the same protocol was performed applying either 1 μ
m
capsaicin alone (n = 7) or 10 m
m
QX-314 alone (n = 7), showing that both must be present for substantial inhibition of sodium channels. C, Block of sodium current by QX-314 added to the intracellular (pipette) solution in whole-cell recording from voltage-clamped DRG neurons. Upper traces, Sodium currents elicited by a step from −80 to 0 mV recorded after 2 min and 12 min in a cell dialyzed by the control intracellular solution. Bottom traces, Same in a cell dialyzed with intracellular solution containing 100 μ
m
QX-314. D, Collected results (mean ± SD) in nine cells with control solution, five cells dialyzed with 50 μ
m
QX-314, and three cells with 100 μ
m
QX-314.
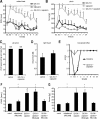
Figure 3.
Effects of QX-314, its combination with capsaicin, and of capsaicin alone on amplitude and latency of the C-fiber and A-fiber Compound Action Potential (CAP) in C57BL\6 and TRPV1−/− mice. Isolated mouse sciatic nerves were superfused with QX-314 (300 μ
m
) and/or capsaicin (10 μ
m
) and CAPs recorded after electrical stimulation. A, Representative trace of the amplitude (upper) and latency (lower) of the C-fiber and A-fiber CAP after superfusion with QX-314 followed by capsaicin. B, Same as in A but after superfusion with capsaicin only. C, Statistical analysis and comparison of CAP amplitudes from recordings after perfusion with QX-314 and capsaicin or capsaicin alone at the indicated time points from recordings as shown in A and B in C57BL\6 (black bars) and TRPV1−/− (white bar inserts) mice. D, Statistical analysis and comparison of CAP latencies from recordings as shown in A and B in C57BL\6 (black bars) and TRPV1−/− (white bar inserts) mice. Data represent the means ± SEM, n = 6–9 C57BL\6; 3 TRPV1−/−. Significance *p < 0.05 calculated by Wilcoxon matched pairs test for intraindividual; #p < 0.01 calculated by Mann–Whitney U test for group comparisons. cap., capsaicin.
Figure 4.
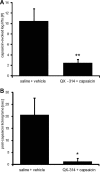
Effects of axonal silencing of TRPV1-expressing sensory neurons on acute nociceptive responses elicited by intraplantar injection of capsaicin. A, B, One hour after silencing TRPV1-expressing neurons by injection of 200 μl of 0.5% QX-314 followed by 200 μl of 0.05% capsaicin (controls received saline followed by vehicle) into the sciatic notch intraplantar capsaicin (0.005%, 20 μl) was injected into the hindpaw and over a 15 min observation period, the number of paw lifts (A) or the total duration of licking bouts (B) was analyzed. Data represent the average ± SEM from six animals per group. Significance was calculated by two-tailed Student's t test. *p < 0.05 and **p < 0.01 compared with saline and vehicle injection.
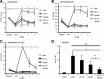
Figure 5.
Sensory processing of TRPV1-expressing neurons in naive rats. Behavioral responses were determined after silencing TRPV1-expressing neurons by injection of 200 μl of 0.5% QX-314 followed by 200 μl of 0.05% capsaicin into the sciatic notch. Different sensory stimulations were applied to the lateral paw. A, Thermal thresholds after radiant heat stimuli. At the indicated time points, paw withdrawal latencies were determined using the Hargreaves test. B, Mechanical thresholds after nociceptive stimulation with an increasing force using a pincher. C, Nociceptive responses after mechanical stimuli induced by a pinprick at 1 h after perineural injections. 6/6, Six rats of six showed a rapid withdrawal response. D, Paw withdrawal responses to light touch stimulations with a brush. Average number of responses to five stimulations to the lateral plantar paw was determined at 1 h after perineural injections. E, Motor function. Toe-spread reflexes were scored after perineural injection of lidocaine [2%] or silencing TRPV1-expressing neurons as above: 0, no spreading; 1, intermediate spreading; 2, full spreading. F, G, RR dependence of silencing TRPV1-expressing axons. Rats received perineural injections of 0.5% QX-314 followed by 0.05% capsaicin and ± the indicated RR concentrations. After 1 h behavioral responses to thermal or mechanical stimuli (F, Hargreaves test as in A; G, pinch test as in B) were determined. In all experiments, data represent the average ± SEM from six animals per group. Significance was calculated by the two-way repeated-measures ANOVA and Bonferroni correction. *p < 0.05 compared with saline and vehicle injection. The dashed line indicates the cutoff. PWL, Paw Withdrawal Latency.
Figure 6.
TRPV1-expressing neurons process heat, mechanical, and cold hyperalgesia during inflammation. At 48 h after intraplantar injection of 100 μl of CFA, TRPV1-expressing neurons were silenced by injection of 200 μl of 0.5% QX-314 followed by 200 μl of 0.05% capsaicin into the sciatic notch and then behavioral responses determined. A, Heat hyperalgesia. Thermal thresholds were determined after radiant heat stimulation to the lateral paw in the Hargreaves test. B, Mechanical hyperalgesia. Mechanical thresholds were determined after stimulation of the lateral paw with an increasing force by a pincher. C, Tactile allodynia. Mechanical thresholds were determined after stimulating the lateral paw with von Frey filaments. D, Cold hyperalgesia. The number of flinches observed during 5 min on a 5°C cold plate was counted at 1 h after silencing. Data represent the average ± SEM from 6 to 12 rats per group. In all temporal courses, significance was calculated by the two-way repeated-measures ANOVA and Bonferroni correction. *p < 0.05 compared with CFA without perineural injections. The dashed line indicates the cutoff. Cold plate experiments were analyzed using a one-way ANOVA followed by Bonferroni post hoc test.
Figure 7.
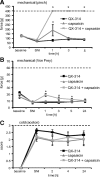
TRPV1-expressing neurons do not mediate cold allodynia in the SNI model. At day 8 after SNI surgery, TRPV1-expressing neurons were silenced by injection of 200 μl of 0.5% QX-314 followed by 200 μl of 0.05% capsaicin into the sciatic notch and behavioral responses determined after sensory stimulation to the lateral paw. A, Mechanical hyperalgesia. Mechanical thresholds were determined after stimulation with an increasing force by a pincher. B, Tactile allodynia. Mechanical thresholds were determined by the von Frey test. C, Cold allodynia. Nociceptive responses induced by a drop of acetone were determined. Data represent the average ± SEM from 6 to 10 rats per group. Significance was calculated by the two-way repeated-measures ANOVA and Bonferroni correction. *p < 0.05 compared with SNI without perineural injections. The dashed line indicates the cutoff.
Similar articles
- Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers.
Binshtok AM, Bean BP, Woolf CJ. Binshtok AM, et al. Nature. 2007 Oct 4;449(7162):607-10. doi: 10.1038/nature06191. Nature. 2007. PMID: 17914397 - Emulsified isoflurane enhances thermal transient receptor potential vanilloid-1 channel activation-mediated sensory/nociceptive blockade by QX-314.
Zhou C, Liang P, Liu J, Zhang W, Liao D, Chen Y, Chen X, Li T. Zhou C, et al. Anesthesiology. 2014 Aug;121(2):280-9. doi: 10.1097/ALN.0000000000000236. Anesthesiology. 2014. PMID: 24667830 - Acid solution is a suitable medium for introducing QX-314 into nociceptors through TRPV1 channels to produce sensory-specific analgesic effects.
Liu H, Zhang HX, Hou HY, Lu XF, Wei JQ, Wang CG, Zhang LC, Zeng YM, Wu YP, Cao JL. Liu H, et al. PLoS One. 2011;6(12):e29395. doi: 10.1371/journal.pone.0029395. Epub 2011 Dec 28. PLoS One. 2011. PMID: 22216270 Free PMC article. - Capsaicin and sensory neurones: a historical perspective.
Szolcsányi J. Szolcsányi J. Prog Drug Res. 2014;68:1-37. doi: 10.1007/978-3-0348-0828-6_1. Prog Drug Res. 2014. PMID: 24941663 Review. - Differential effects of TRPV channel block on polymodal activation of rat cutaneous nociceptors in vitro.
St Pierre M, Reeh PW, Zimmermann K. St Pierre M, et al. Exp Brain Res. 2009 Jun;196(1):31-44. doi: 10.1007/s00221-009-1808-3. Epub 2009 Apr 30. Exp Brain Res. 2009. PMID: 19404626 Review.
Cited by
- Pharmacological Inhibition of Voltage-gated Ca(2+) Channels for Chronic Pain Relief.
Lee S. Lee S. Curr Neuropharmacol. 2013 Dec;11(6):606-20. doi: 10.2174/1570159X11311060005. Curr Neuropharmacol. 2013. PMID: 24396337 Free PMC article. - Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade.
Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T, Wang F, Oh SB, Ji RR. Xu ZZ, et al. Nat Med. 2015 Nov;21(11):1326-31. doi: 10.1038/nm.3978. Epub 2015 Oct 19. Nat Med. 2015. PMID: 26479925 Free PMC article. - Automated and rapid self-report of nociception in transgenic mice.
Black CJ, Allawala AB, Bloye K, Vanent KN, Edhi MM, Saab CY, Borton DA. Black CJ, et al. Sci Rep. 2020 Aug 6;10(1):13215. doi: 10.1038/s41598-020-70028-8. Sci Rep. 2020. PMID: 32764714 Free PMC article. - Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain.
Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Lippoldt EK, et al. J Neurosci. 2013 Jul 24;33(30):12543-52. doi: 10.1523/JNEUROSCI.5765-12.2013. J Neurosci. 2013. PMID: 23884957 Free PMC article. - Primary sensory neuron-specific interference of TRPV1 signaling by AAV-encoded TRPV1 peptide aptamer attenuates neuropathic pain.
Xiang H, Liu Z, Wang F, Xu H, Roberts C, Fischer G, Stucky C, Caron D, Pan B, Hogan Q, Yu H. Xiang H, et al. Mol Pain. 2017 Jan-Dec;13:1744806917717040. doi: 10.1177/1744806917717040. Mol Pain. 2017. PMID: 28604222 Free PMC article.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–879. - PMC - PubMed
- Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol. 2010;298:C1457–C1468. - PubMed
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS064274/NS/NINDS NIH HHS/United States
- R01 NS039518/NS/NINDS NIH HHS/United States
- R01 NS064274/NS/NINDS NIH HHS/United States
- R37 NS039518/NS/NINDS NIH HHS/United States
- P01 NS072040/NS/NINDS NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- NS072040/NS/NINDS NIH HHS/United States
- T32 MH020017/MH/NIMH NIH HHS/United States
- R01 NS038253/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical