The anti-inflammatory effects of matrix metalloproteinase-3 on irreversible pulpitis of mature erupted teeth - PubMed (original) (raw)
The anti-inflammatory effects of matrix metalloproteinase-3 on irreversible pulpitis of mature erupted teeth
Hisanori Eba et al. PLoS One. 2012.
Abstract
Matrix metalloproteinases (MMPs) are involved in extracellular matrix degradation and the modulation of cell behavior. These proteinases have also been implicated in tissue repair and regeneration. Our previous studies have demonstrated that MMP-3 elicits stimulatory effects on the proliferation and the migration of endothelial cells as well as anti-apoptotic effects on these cells in vitro. In addition, we found that MMP-3 enhanced the regeneration of lost pulp tissue in a rat incisor pulp injury model. However, continuously erupting rodent incisors exhibit significantly different pulp organization compared with mature erupted teeth. Therefore, we have further extended these studies using a canine irreversible pulpitis model to investigate the effects of MMP-3. In this study, the crowns of the canine mature premolars were removed and the pulp tissues were amputated. The amputated pulp tissues remained exposed for 24 or 72 hours to induce mild or severe irreversible pulpitis, respectively, followed by sealing of the cavities. In both models, the whole pulp tissues became necrotic by day 14. In this mild pulpitis model, the regeneration of pulp tissue with vasculature and nerves was observed until 14 days after sealing with MMP-3, followed by extracellular matrix formation in the regenerated pulp tissues until day 28. The treatment with MMP-3 resulted in a decrease in the number of macrophage and antigen-presenting cells and a significant inhibition of IL-6 expression on day 3. The inhibition of MMP-3 activity abolished these anti-inflammatory effects. Immunofluorescence staining demonstrated that MMP-3 was involved in the modification of serum-derived hyaluronan-associated proteins and hyaluronan (SHAP-HA) complexes possibly through the degradation of versican. These results demonstrate that MMP-3 can act as an anti-inflammatory agent and suggest that MMP-3 might represent a useful therapy for the treatment of mild irreversible pulpitis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
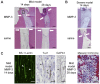
Figure 1. The establishment of mild and severe irreversible pulpitis models.
A. Schematic diagrams of the amputation of the dog mature molar pulp tissues and subsequent cavity sealing. The crowns of the upper and lower premolars were removed, and the pulp tissues were amputated using a round burr. The amputated pulp tissues were exposed to allow for infection and treated with solution absorbed in Spongel. After the treatments, the cavity were sealed with phosphate cement and light-cured composite resin. B. The histology of the pulp tissues from dogs with mild irreversible pulpitis. The left panel shows amputated pulp tissue that remained exposed for 24 hours. After 24 hours, the cavity was covered with spongel and sealed. The right panel shows the pulp at 14 days after sealing. C. The histology of the pulp tissues from dogs with severe irreversible pulpitis. The left panel shows amputated pulp tissue that remained exposed for 72 hours. After 72 hours, the cavity was sealed. The right panel shows the pulp tissue at 14 days after sealing. Scale bar, 500 µm.
Figure 2. The pulp tissue regeneration induced by MMP-3 in the irreversible pulpitis model.
A. H&E staining of pulp tissues from dogs with mild pulpitis at 14 and 28 days after MMP-3 or saline treatment, as indicated. The arrows show the imaginary amputated site, with the indicated areas magnified in C. Scale bar, 200 µm. B. H&E staining of pulp tissues from dogs with severe pulpitis at 14 days after MMP-3 or saline treatment, as indicated. The arrows indicate the imaginary amputated site. Scale bar, 200 µm. C. Immunohistochemical analysis for BS-1-lectin, TuJ1, and GAP43 and Masson’s trichrome staining of pulp tissues from dogs with mild pulpitis at 14 or 28 days after MMP-3 treatment, as indicated. The representative staining of the cells is indicated by arrowheads. Scale bar, 50 µm.
Figure 3. Time course of the histological changes of the pulp tissues from dogs with mild pulpitis.
H&E staining of pulp tissues from the dog with mild pulpitis at the indicated number of days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline alone, as indicated. The arrows indicate the imaginary amputated site. Scale bar, 200 µm.
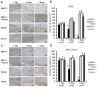
Figure 4. Immunohistochemical analysis of CD68 and MHC class II in samples from dogs with mild pulpitis.
A. Immunostaining of pulp tissues using anti-CD68 IgG on the indicated days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline alone, as indicated. Scale bar, 100 µm. B. Quantitative analysis of the CD68-positive cells on the indicated days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline alone, as indicated. Error bars, ± SEM, **P<0.01. C. Immunostaining of pulp tissues from dogs with mild pulpitis using anti-MHC class II IgG on the indicated days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline alone, as indicated. Scale bar, 100 µm. D. Quantitative analysis of the MHC class II-positive cells on the indicated days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline alone, as indicated. Error bars, ± SEM, **P<0.01.
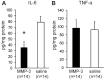
Figure 5. Levels of IL-6 and TNF-α at 3 days after MMP-3 treatment in mild pulpitis model.
Homogenates of pulp tissues were prepared at 3 days after MMP-3 or saline treatment as indicated. A. The average concentration (ng/mg protein) of IL-6 is indicated. B. The average concentration (ng/mg protein) of TNF-α is indicated. Error bars, ± SEM, *P<0.05.
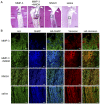
Figure 6. Hyaluronan (HA), SHAP and versican localization in the mild pulpitis model.
Immunofluorescence-stained pulp tissues were prepared 3 days after treatment. A. H&E-stained pulp tissues from dogs with mild pulpitis 3 days after treatment with MMP-3, MMP-3 plus NNGH, NNGH alone or saline as indicated, with the indicated area magnified in B. The arrows indicate the imaginary amputated site. Scale bar, 200 µm. B. Pulpitis tissues stained with biotinylated HABP (HA), anti-SHAP (SHAP) and anti-versican as indicated. Scale bar, 20 µm.
Similar articles
- Down-regulation of inflammatory mediator synthesis and infiltration of inflammatory cells by MMP-3 in experimentally induced rat pulpitis.
Takimoto K, Kawashima N, Suzuki N, Koizumi Y, Yamamoto M, Nakashima M, Suda H. Takimoto K, et al. J Endod. 2014 Sep;40(9):1404-9. doi: 10.1016/j.joen.2014.04.001. Epub 2014 Apr 21. J Endod. 2014. PMID: 25146022 - A Prospective Clinical Pilot Study on the Level of Matrix Metalloproteinase-9 in Dental Pulpal Blood as a Marker for the State of Inflammation in the Pulp Tissue.
Mente J, Petrovic J, Gehrig H, Rampf S, Michel A, Schürz A, Pfefferle T, Saure D, Erber R. Mente J, et al. J Endod. 2016 Feb;42(2):190-7. doi: 10.1016/j.joen.2015.10.020. Epub 2015 Dec 24. J Endod. 2016. PMID: 26725178 - Anti-Inflammatory Effects of Melatonin and 5-Methoxytryptophol on Lipopolysaccharide-Induced Acute Pulpitis in Rats.
Kermeoğlu F, Aksoy U, Sebai A, Savtekin G, Özkayalar H, Sayıner S, Şehirli AÖ. Kermeoğlu F, et al. Biomed Res Int. 2021 Feb 12;2021:8884041. doi: 10.1155/2021/8884041. eCollection 2021. Biomed Res Int. 2021. PMID: 33628825 Free PMC article. - Role(s) of cytokines in pulpitis: Latest evidence and therapeutic approaches.
Khorasani MMY, Hassanshahi G, Brodzikowska A, Khorramdelazad H. Khorasani MMY, et al. Cytokine. 2020 Feb;126:154896. doi: 10.1016/j.cyto.2019.154896. Epub 2019 Oct 25. Cytokine. 2020. PMID: 31670007 Review. - Vital pulp therapy of mature permanent teeth with irreversible pulpitis from the perspective of pulp biology.
Lin LM, Ricucci D, Saoud TM, Sigurdsson A, Kahler B. Lin LM, et al. Aust Endod J. 2020 Apr;46(1):154-166. doi: 10.1111/aej.12392. Epub 2019 Dec 21. Aust Endod J. 2020. PMID: 31865629 Review.
Cited by
- Dentin Matrix Metalloproteinases: A Futuristic Approach Toward Dentin Repair and Regeneration.
Agrawal P, Nikhade P, Chandak M, Ikhar A, Bhonde R. Agrawal P, et al. Cureus. 2022 Aug 12;14(8):e27946. doi: 10.7759/cureus.27946. eCollection 2022 Aug. Cureus. 2022. PMID: 36120221 Free PMC article. Review. - Dental pulp mesenchymal stem cells-response to fibrin hydrogel reveals ITGA2 and MMPs expression.
Tong D, Gobert S, Reuzeau A, Farges JC, Leveque M, Bolon M, Costantini A, Pasdeloup M, Lafont J, Ducret M, Bekhouche M. Tong D, et al. Heliyon. 2024 Jun 18;10(13):e32891. doi: 10.1016/j.heliyon.2024.e32891. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027533 Free PMC article. - Biomechanical Modulation of Dental Pulp Stem Cell (DPSC) Properties for Soft Tissue Engineering.
Gross T, Dieterle MP, Vach K, Altenburger MJ, Hellwig E, Proksch S. Gross T, et al. Bioengineering (Basel). 2023 Mar 3;10(3):323. doi: 10.3390/bioengineering10030323. Bioengineering (Basel). 2023. PMID: 36978714 Free PMC article. - Dentine matrix metalloproteinases as potential mediators of dentine regeneration.
Guirado E, George A. Guirado E, et al. Eur Cell Mater. 2021 Nov 24;42:392-400. doi: 10.22203/eCM.v042a24. Eur Cell Mater. 2021. PMID: 34818431 Free PMC article. Review. - Matrix metalloproteinase-3 in odontoblastic cells derived from ips cells: unique proliferation response as odontoblastic cells derived from ES cells.
Hiyama T, Ozeki N, Mogi M, Yamaguchi H, Kawai R, Nakata K, Kondo A, Nakamura H. Hiyama T, et al. PLoS One. 2013 Dec 16;8(12):e83563. doi: 10.1371/journal.pone.0083563. eCollection 2013. PLoS One. 2013. PMID: 24358294 Free PMC article. Retracted.
References
- Staquet MJ, Carrouel F, Keller JF, Baudouin C, Msika P, et al. (2011) Pattern-recognition receptors in pulp defense. Adv Dent Res 23: 296–301. - PubMed
- Hahn CL, Liewehr FR (2007) Innate immune responses of the dental pulp to caries. J Endod 33: 643–651. - PubMed
- Zero DT, Zandona AF, Vail MM, Spolnik KJ (2011) Dental caries and pulpal disease. Dent Clin North Am 55: 29–46. - PubMed
- Torabinejad M, Walton R (2008) Endodntics: Principles and Practice.Amsterdam: Elsevier Health Sciences.
- Piwowarczyk A, Lauer HC, Sorensen JA (2005) Microleakage of various cementing agents for full cast crowns. Dent Mater 21: 445–453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous