Targeting GRB7/ERK/FOXM1 signaling pathway impairs aggressiveness of ovarian cancer cells - PubMed (original) (raw)
Targeting GRB7/ERK/FOXM1 signaling pathway impairs aggressiveness of ovarian cancer cells
David W Chan et al. PLoS One. 2012.
Erratum in
- PLoS One. 2014;9(10):e110304
Abstract
Ovarian cancer is a highly lethal disease with poor prognosis and especially in high-grade tumor. Emerging evidence has reported that aberrant upregulation and activation of GRB7, ERK as well as FOXM1 are closely associated with aggresivenesss of human cancers. However, the interplay between these factors in the pathogenesis of human cancers still remains unclear. In this study, we found that GRB7 (P<0.0001), ERK phosphorylation (P<0.0001) and FOXM1 (P = 0.001) were frequently increased and associated with high-grade tumors, as well as a high tendency in association with advanced stage ovarian cancer by immunohistochemical analysis. Intriguingly, the expressions of GRB7 (P<0.0001), ERK phosphorylation (P<0.001) and FOXM1 (P<0.001) showed a significant stepwise increase pattern along Grade 1 to Grade 3 ovarian cancers. Biochemical studies using western blot analysis demonstrated that enforced expression or knockdown of GRB7 showed GRB7 could elevate the levels of ERK phosphorylation and FOXM1, whereas enforced expression of FOXM1 could not alter levels of GRB7 and ERK phosphorylation. But inhibition of ERK signaling by U0126 or PD98059 could reduce the level of FOXM1 in GRB7-overexpressing ovarian cancer cells, suggesting that GRB7, ERK and FOXM1 are regulated orderly. Moreover, inhibition of ERK activity by U0126 or PD98059, or decreased FOXM1 expression by Thiostrepton significantly inhibited cell migration/invasion, tumor growth in vitro and in vivo. Collectively, our findings confer that targeting GRB7/ERK/FOXM1 signaling cascade may be a promising molecular therapeutic choice in combating ovarian cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
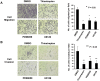
Figure 1. A significant stepwise increase in the expressions of GRB7, ERK phosphorylation and FOXM1 along ovarian tumor grade.
(A) The expressions of GRB7, ERK phosphorylation and FOXM1 were evaluated by immunohistochemical analysis using specific antibodies on an ovarian cancer tissue array (OVC1021, Pantomics). (B) Representative pictures show the stepwise increase of GRB7, ERK phosphorylation and FOXM1 expressions from Grade 1 to Grade 3 ovarian cancer (serous subtype) (200× magnifications).
Figure 2. GRB7/ERK/FOXM1 was regulated in the same signaling axis.
(A) Treatment with U0126 (5 µM) showed a significant reduction in the expression of ERK phosphorylation accompanied with FOXM1 in ovarian cancer cells time dependently, whereas no change in GRB7 expression was found in A2780cp cells. (B) Treatment of Thiostrepton (20 µM) remarkably reduced the expression of FOXM1 only but no change in the expressions of GRB7 and ERK phosphorylation in A2780cp cells (left). Depletion of FOXM1 by siRNA knockdown did not alter the expression of GRB7 and ERK phosphorylation (right). C, siRNA scrambled control. si1, si2 and si3 siRNAs targeting three different regions of human FOXM1 and knockdown the expression of FOXM1 by 60%, 45% and 70% respectively. (C) Two out of four GRB7 shRNA constructs (sh1 and sh2) showed ∼70% knockdown of GRB7 accompanied with a reduction of ERK phosphorylation and FOXM1 expressions in OVCA433 cells. The scrambled control (NC) was used as negative control. (D) Enforced expression of GRB7 increased ERK phosphorylation and FOXM1. However, treatment with either U0126 (10 µM) or PD98059 (20 µM) could suppress the induced ERK phosphorylation and FOXM1 in A2780cp and OVCA433 ovarian cancer cells.
Figure 3. Inhibition of ERK phosphorylation or FOXM1 significantly decreased both migration and invasion of GRB7-overexpressing ovarian cancer cells.
OVCA433 cells with stable expression of GFP/GRB7 (OVCA433-GRB7) were treated with DMSO as control, Thiostrepton (20 µM), PD98059 (20 µM) and U0126 (10 µM) for 6 hours and were analyzed by (A) Transwell cell migration assay. The representative pictures and bar chart showed significant reduction in the number of migratory cells through Matrigel-coated membrane in OVCA433-GRB7 cells treated with Thiostrepton, PD98059 and U0126 than DMSO control (*P<0.02, Student _t_-test) at 8-hour; (B) Transwell cell invasion assay. The representative pictures and bar chart showed significant reduction in the invasion rate in OVCA433-GRB7 cells treated with Thiostrepton, PD98059 and U0126 when compared with DMSO control (*P<0.05, Student _t_-test) at 15-hour.
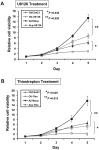
Figure 4. Inhibition of either ERK phosphorylation or FOXM1 expression impaired cell growth in ovarian cancer cells.
(A) XTT cell proliferation assays showed that the inhibition of ERK phosphorylation by U0126 (10 µM) significantly abrogated the cell proliferation rate in GRB7 stably expressing OVCA433 cells (P = 0.020, Student _t_-test) and A2780cp cells (P = 0.030, Student _t_-test) as compared with their vector controls. (B) XTT cell proliferation assays showed that the suppression of FOXM1 expression by Thiostrepton (20 µM) significantly reduced the cell proliferation rate in GRB7 stably expressing OVCA433 cells (P = 0.015, Student _t_-test) and A2780cp cells (P = 0.025, Student _t_-test) as compared with their vector controls.
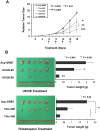
Figure 5. Inhibition of ERK phosphorylation or FOXM1 expression reduced tumor growth in a mouse xenograft model.
(A) The GRB7 stably expressing A2780cp cells (Acp-GRB7) were subcutaneously injected into the right flank of nude mice. Mice were divided into 5 groups (5 mice per group) and treated with either DMSO as a control, or U0126 (25 or 50 µM/kg) or Thiostrepton (200 or 300 µM/kg) for every 3-day since on day 6 (Arrows represent the injections). The relative tumor size was calculated relative to those of the first day of treatment (day 0) and are represented as relative mean size (%)±SE for each group (*P = 0. 032, **P<0.01, and ***P = 0. 005, are significantly different from the DMSO control group, Student _t_-test). (B) The representative pictures and bar charts show the average tumor weight of each group taken on day 18. (*P = 0. 043, **P = 0. 001, and ***P<0.02, are significantly different from the DMSO control group, Student _t_-test).
Similar articles
- Aberrant activation of ERK/FOXM1 signaling cascade triggers the cell migration/invasion in ovarian cancer cells.
Lok GT, Chan DW, Liu VW, Hui WW, Leung TH, Yao KM, Ngan HY. Lok GT, et al. PLoS One. 2011;6(8):e23790. doi: 10.1371/journal.pone.0023790. Epub 2011 Aug 17. PLoS One. 2011. PMID: 21858223 Free PMC article. - Methylation-associated silencing of miR-193a-3p promotes ovarian cancer aggressiveness by targeting GRB7 and MAPK/ERK pathways.
Chen K, Liu MX, Mak CS, Yung MM, Leung TH, Xu D, Ngu SF, Chan KK, Yang H, Ngan HY, Chan DW. Chen K, et al. Theranostics. 2018 Jan 1;8(2):423-436. doi: 10.7150/thno.22377. eCollection 2018. Theranostics. 2018. PMID: 29290818 Free PMC article. - Differential functions of growth factor receptor-bound protein 7 (GRB7) and its variant GRB7v in ovarian carcinogenesis.
Wang Y, Chan DW, Liu VW, Chiu P, Ngan HY. Wang Y, et al. Clin Cancer Res. 2010 May 1;16(9):2529-39. doi: 10.1158/1078-0432.CCR-10-0018. Epub 2010 Apr 13. Clin Cancer Res. 2010. PMID: 20388850 - FOXM1: A Multifunctional Oncoprotein and Emerging Therapeutic Target in Ovarian Cancer.
Liu C, Barger CJ, Karpf AR. Liu C, et al. Cancers (Basel). 2021 Jun 19;13(12):3065. doi: 10.3390/cancers13123065. Cancers (Basel). 2021. PMID: 34205406 Free PMC article. Review. - Grb7 knockout mice develop normally but litters born to knockout females fail to thrive.
Lofgren KA, Kenny PA. Lofgren KA, et al. Dev Dyn. 2024 Jul;253(7):677-689. doi: 10.1002/dvdy.686. Epub 2023 Dec 23. Dev Dyn. 2024. PMID: 38140940 Review.
Cited by
- Flow-induced Shear Stress Confers Resistance to Carboplatin in an Adherent Three-Dimensional Model for Ovarian Cancer: A Role for EGFR-Targeted Photoimmunotherapy Informed by Physical Stress.
Nath S, Pigula M, Khan AP, Hanna W, Ruhi MK, Dehkordy FM, Pushpavanam K, Rege K, Moore K, Tsujita Y, Conrad C, Inci F, Carmen MGD, Franco W, Celli JP, Demirci U, Hasan T, Huang HC, Rizvi I. Nath S, et al. J Clin Med. 2020 Mar 28;9(4):924. doi: 10.3390/jcm9040924. J Clin Med. 2020. PMID: 32231055 Free PMC article. - FOXM1 is a downstream target of LPA and YAP oncogenic signaling pathways in high grade serous ovarian cancer.
Fan Q, Cai Q, Xu Y. Fan Q, et al. Oncotarget. 2015 Sep 29;6(29):27688-99. doi: 10.18632/oncotarget.4280. Oncotarget. 2015. PMID: 26299613 Free PMC article. - Bitter Melon (Momordica charantia) Extract Inhibits Tumorigenicity and Overcomes Cisplatin-Resistance in Ovarian Cancer Cells Through Targeting AMPK Signaling Cascade.
Yung MM, Ross FA, Hardie DG, Leung TH, Zhan J, Ngan HY, Chan DW. Yung MM, et al. Integr Cancer Ther. 2016 Sep;15(3):376-89. doi: 10.1177/1534735415611747. Epub 2015 Oct 19. Integr Cancer Ther. 2016. PMID: 26487740 Free PMC article. - Evaluation of Cyclic Peptide Inhibitors of the Grb7 Breast Cancer Target: Small Change in Cargo Results in Large Change in Cellular Activity.
Sang J, Kulkarni K, Watson GM, Ma X, Craik DJ, Henriques ST, Poth AG, Benfield AH, Wilce JA. Sang J, et al. Molecules. 2019 Oct 17;24(20):3739. doi: 10.3390/molecules24203739. Molecules. 2019. PMID: 31627265 Free PMC article. - MiR-214 inhibits cell migration, invasion and promotes the drug sensitivity in human cervical cancer by targeting FOXM1.
Wang JM, Ju BH, Pan CJ, Gu Y, Li MQ, Sun L, Xu YY, Yin LR. Wang JM, et al. Am J Transl Res. 2017 Aug 15;9(8):3541-3557. eCollection 2017. Am J Transl Res. 2017. PMID: 28861147 Free PMC article.
References
- Bristow RE, Palis BE, Chi DS, Cliby WA (2010) The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol 118: 262–267. - PubMed
- D’Alo D, Stracci F, Cassetti T, Scheibel M, Pascucci C, et al. (2010) Recent trends in incidence, mortality and survival after cancer of the female breast and reproductive organs. Umbria, Italy: 1978–2005. Eur J Gynaecol Oncol 31: 174–180. - PubMed
- O’Neill CJ, Deavers MT, Malpica A, Foster H, McCluggage WG (2005) An immunohistochemical comparison between low-grade and high-grade ovarian serous carcinomas: significantly higher expression of p53, MIB1, BCL2, HER-2/neu, and C-KIT in high-grade neoplasms. Am J Surg Pathol 29: 1034–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was supported by the Wong Check She Charitable Foundation. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous