Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography - PubMed (original) (raw)
Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography
Arash Kamali et al. Brain Struct Funct. 2014 Jan.
Abstract
The major language pathways such as superior longitudinal fasciculus (SLF) pathways have been outlined by experimental and diffusion tensor imaging (DTI) studies. The SLF I and some of the superior parietal lobule connections of the SLF pathways have not been depicted by prior DTI studies due to the lack of imaging sensitivity and adequate spatial resolution. In the current study, the trajectory of the SLF fibers has been delineated on five healthy human subjects using diffusion tensor tractography on a 3.0-T scanner at high spatial resolution. We also demonstrate for the first time the trajectory and connectivity of the SLF fibers in relation to other language pathways as well as the superior parietal lobule connections of the language circuit using high spatial resolution DTI in the healthy adult human brain.
Figures
Figure1
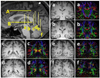
The selected ROI seeds (ROI 1 and ROI 2) for each language fiber tract are illustrated. The detailed description of the planes and ROIs for each tract is in the methods section. (a) SLF I: the ROI 1 is delineated over the green association bundles just superolateral to the cingulum. The ROI 2 was placed over the fibers generated on the superolateral aspect of the cingulum. (b) SLF II: the ROI 1 is placed over the white matter of the angular gyrus (coronal plane e in Figure 2). The second ROI was placed on the fibers generated on the association green area. (c) SLF III: the ROI 1 is paced over the periventricular green association fibers followed by the second ROI over the fibers generated on the white matter of the supramarginal gyrus (d) AF: the first ROI is the same as ROI 1 for the SLF III. The ROI 2 is situated over the periventricular craniocaudally oriented blue fibers. (e) SLF TP-IPL: the first ROI was selected on the periventricular blue fibers lateral to the green association fibers followed by seeding the second ROI on the fibers generated on the inferior parietal lobule. (f) SLF TP-SPL: the first ROI is the same as ROI 1 for SLF TP-IPL. The ROI 2 is seeded over the fibers generated at the centrum semiovale. (g) MdLF-SPL: the ROI 1 is selected over the ascending blue fibers of the parietal cortex and the ROI 2 on the fibers generated in the superior temporal gyrus. (h) MdLF-IPL: the ROI1 was selected on the superior temporal gyrus, followed by placing the second ROI on the fibers generated at the parietooccipital confluence.
Figure 2
Structural (T1-weighted) MRI of the sagittal midline plane showing the corresponding axial and coronal planes used for ROI placement in figure 1. The corresponding T1-weighted and diffusion tensor color-coded map of the coronal sections of a–f are also provided. (a) The coronal plane a is at the midthalamic, coronal plane b is at the posterior-most portion of the fornix on the midline plane, coronal plane c is at the posterior-most coronal level of the corpus callosum, coronal plane d is at the confluence of the superior ramus and the cingulate sulcus, coronal plane e is at the cortical origin of the ramus of the cingulate sulcus and the coronal plane f is at the caudal end of the parietooccipital sulcus in the midsagittal cut. (b) The axial plane A is at the level of the cingulate sulcus, axial plane B is at the level of the anterior commissure.
Figure 3
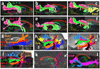
(3a–e) Three-dimensional reconstructions of the SLF I (light blue), SLF III (green), MdLF (inferior parietal lobule connection in red and superior parietal lobule connection in orange color), ILF (dark blue) and Cingulum (Cing in yellow) for five individuals were portrayed. (3f) The arcuate fasciculus is shown in maroon color in one of the subjects. (3g–l) The SLF I (light blue) and the cingulum (yellow) are imaged in five subjects.
Figure 4
(a–f) Three-dimensional reconstructions of the SLF II (pink), and SLF III (green) created for each individual are shown. Trajectory of the extreme capsule (EmC) (yellow in 4c) as well as the relationship of the SLF II and SLF III with extreme capsule in one of the subjects is shown in Figure 4c. (g) The inferior parietal lobule connection of the MdLF (MdLF-IPL in red color) and the superior parietal lobule connection of the MdLF (MdLF-SPL in orange color) and the ILF (dark blue) are demonstrated. (4h–l) The relationship of the SLF II and SLF III with other adjacent association fibers such as the cingulum (yellow), SLF I (light blue), ILF (dark blue), AF (black) and temporoparietal portion of the SLF (SLF TP in purple color) are illustrated. In figure 4h the SLF TP-IPL and SLF TP-IPL are combined together and are assigned the orange color. (k) The trajectory of the SLF TP is portrayed. The angular gyrus (inferior parietal lobule) connections of the SLF TP are demonstrated by the arrows (h–k). The superior parietal connections are also shown. (l) The IFOF (pink) and the UF (green) in one of the subjects are illustrated.
Figure 5
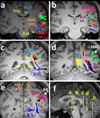
Five consecutive coronal T1 images show a detailed description of the language pathways. Please attention that the color-coding is fixed across all the images. The coarse trajectories of the SLF I (light blue), SLF II (pink), SLF III (green), SLF TP (purple), MdLF (inferior parietal lobule connection in red and superior parietal lobule connection in orange color), ILF (dark blue), Cingulum (yellow) and AF (black) for one individual are illustrated. Corresponding axial planes are demonstrated in mid sagittal cut in Figure 5f.
Similar articles
- Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain.
Kamali A, Sair HI, Radmanesh A, Hasan KM. Kamali A, et al. Neuroscience. 2014 Sep 26;277:577-83. doi: 10.1016/j.neuroscience.2014.07.035. Epub 2014 Jul 30. Neuroscience. 2014. PMID: 25086308 - The anatomical characteristics of superior longitudinal fasciculus I in human brain: Diffusion tensor tractography study.
Jang SH, Hong JH. Jang SH, et al. Neurosci Lett. 2012 Jan 6;506(1):146-8. doi: 10.1016/j.neulet.2011.10.069. Epub 2011 Nov 6. Neurosci Lett. 2012. PMID: 22085696 - Identifying preoperative language tracts and predicting postoperative functional recovery using HARDI q-ball fiber tractography in patients with gliomas.
Caverzasi E, Hervey-Jumper SL, Jordan KM, Lobach IV, Li J, Panara V, Racine CA, Sankaranarayanan V, Amirbekian B, Papinutto N, Berger MS, Henry RG. Caverzasi E, et al. J Neurosurg. 2016 Jul;125(1):33-45. doi: 10.3171/2015.6.JNS142203. Epub 2015 Dec 11. J Neurosurg. 2016. PMID: 26654181 - Tractography and the connectome in neurosurgical treatment of gliomas: the premise, the progress, and the potential.
Henderson F, Abdullah KG, Verma R, Brem S. Henderson F, et al. Neurosurg Focus. 2020 Feb 1;48(2):E6. doi: 10.3171/2019.11.FOCUS19785. Neurosurg Focus. 2020. PMID: 32006950 Free PMC article. Review. - Subcortical anatomy of the lateral association fascicles of the brain: A review.
Martino J, De Lucas EM. Martino J, et al. Clin Anat. 2014 May;27(4):563-9. doi: 10.1002/ca.22321. Epub 2014 Jan 22. Clin Anat. 2014. PMID: 24453050 Review.
Cited by
- White Matter Correlates of Theory of Mind in Patients With First-Episode Psychosis.
Kim NS, Lee TY, Hwang WJ, Kwak YB, Kim S, Moon SY, Lho SK, Oh S, Kwon JS. Kim NS, et al. Front Psychiatry. 2021 Mar 5;12:617683. doi: 10.3389/fpsyt.2021.617683. eCollection 2021. Front Psychiatry. 2021. PMID: 33746794 Free PMC article. - Magnetic resonance markers of tissue damage related to connectivity disruption in multiple sclerosis.
Solana E, Martinez-Heras E, Martinez-Lapiscina EH, Sepulveda M, Sola-Valls N, Bargalló N, Berenguer J, Blanco Y, Andorra M, Pulido-Valdeolivas I, Zubizarreta I, Saiz A, Llufriu S. Solana E, et al. Neuroimage Clin. 2018 Jul 12;20:161-168. doi: 10.1016/j.nicl.2018.07.012. eCollection 2018. Neuroimage Clin. 2018. PMID: 30094165 Free PMC article. - A Connectomic Atlas of the Human Cerebrum-Chapter 10: Tractographic Description of the Superior Longitudinal Fasciculus.
Conner AK, Briggs RG, Rahimi M, Sali G, Baker CM, Burks JD, Glenn CA, Battiste JD, Sughrue ME. Conner AK, et al. Oper Neurosurg (Hagerstown). 2018 Dec 1;15(suppl_1):S407-S422. doi: 10.1093/ons/opy264. Oper Neurosurg (Hagerstown). 2018. PMID: 30260421 Free PMC article. - Retained executive abilities in mild cognitive impairment are associated with increased white matter network connectivity.
Farrar DC, Mian AZ, Budson AE, Moss MB, Koo BB, Killiany RJ; Alzheimer’s Disease Neuroimaging Initiative. Farrar DC, et al. Eur Radiol. 2018 Jan;28(1):340-347. doi: 10.1007/s00330-017-4951-4. Epub 2017 Jul 10. Eur Radiol. 2018. PMID: 28695358 Free PMC article. - Subtle motor signs in children with ADHD and their white matter correlates.
Hyde C, Fuelscher I, Rosch KS, Seymour KE, Crocetti D, Silk T, Singh M, Mostofsky SH. Hyde C, et al. Hum Brain Mapp. 2024 Oct;45(14):e70002. doi: 10.1002/hbm.70002. Hum Brain Mapp. 2024. PMID: 39365253 Free PMC article.
References
- Barrick TR, Clark CA. Singularities in diffusion tensor fields and their relevance in white matter fiber tractography. Neuroimage. 2004;22:481–491. - PubMed
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. - PubMed
- Bernal B, Altman N. The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging. 2010;28:217–225. - PubMed
- Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18:1094–1111. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS052505/NS/NINDS NIH HHS/United States
- S10 RR019186/RR/NCRR NIH HHS/United States
- S10 RR19186/RR/NCRR NIH HHS/United States
- R01-NS052505-04/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources