Virus-induced ER stress and the unfolded protein response - PubMed (original) (raw)
Virus-induced ER stress and the unfolded protein response
Lingrui Zhang et al. Front Plant Sci. 2012.
Abstract
The accumulation of unfolded or misfolded proteins in the lumen of the endoplasmic reticulum (ER) results in ER stress that triggers cytoprotective signaling pathways, termed the unfolded protein response (UPR), to restore and maintain homeostasis in the ER or to induce apoptosis if ER stress remains unmitigated. The UPR signaling network encompasses three core elements, i.e., PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring protein-1 (IRE1). Activation of these three branch pathways of the UPR leads to the translation arrest and degradation of misfolded proteins, the expression of ER molecular chaperones, and the expansion of the ER membrane to decrease the load of proteins and increase the protein-folding capacity in the ER. Recently, the essential roles of the UPR have been implicated in a number of mammalian diseases, particularly viral diseases. In virus-infected cells, the cellular translation machinery is hijacked by the infecting virus to produce large amounts of viral proteins, which inevitably perturbs ER homeostasis and causes ER stress. This review summarizes current knowledge about the UPR signaling pathways, highlights two identified UPR pathways in plants, and discuss progress in elucidating the UPR in virus-infected cells and its functional roles in viral infection.
Keywords: ER stress; endoplasmic reticulum; signaling transduction; unfolded protein response; virus.
Figures
Figure 1
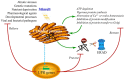
ER stress and UPR functions. Disturbances of ER homeostasis cause overload of unfolded or misfolded protein in the ER lumen, a condition termed ER stress, triggering the UPR. The UPR may be induced by pharmacological chemicals, such as tunicamycin, thapsigargin, homocysteine, reductive/oxidative agents as well as non-steroidal anti-inflammatory agents, which impose stress on the ER by causing the vigorous protein synthesis, the imbalance of ER Ca2+ and redox, and the inhibition of protein modification or transfer to the Golgi body. In mammalian cells, ER stress also occurs under many circumstances, such as nutrient deprivation, developmental processes, genetic mutation, as well as pathogenic insult. The best-known example of ER stress arising from genetic mutation is the protein-misfolding diseases in human. Recent reports in plants have indicated a close connection between the UPR and environmental stimuli such as heat, salt, and drought stress as well as viral attack, although the underlying mechanisms are largely unknown. The purpose of the induced UPR is to restore the ER function and relive the stress exerted on the ER. In addition, the UPR also eliminates the cytotoxic malformed proteins, which are dislocated across the ER membrane for ubiquitination (Ub) and proteasome-mediated degradation through a pathway known as ERAD. However, if ER homeostasis or function cannot be re-established, programmed cell death will be activated by the UPR, presumably to protect the organism from the rogue cells that display misfolded proteins, which has not yet been confirmed in plants and is not shown in the diagram.
Figure 2
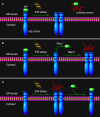
ER stress sensing mechanism by IRE1/PERK. Three models are proposed to explain IRE1/PERK activation in response to the accumulation of unfolded proteins in the ER lumen. (A) The indirect recognition model proposes that BiP binding maintains IRE1/PERK in an inactive monomeric state. During ER stress, BiP is dissociated from its partners to bind unfolded proteins, which leads to the spontaneous dimerization of IRE1/PERK and activation of their RNase domains. In this case, BiP operates as the “UPR master control/ER stress sensor.” The model may also operate in the control of ATF6 activation. (B) The semi-direct recognition model summarizes findings from studies of IRE1p in yeast and analyses of IRE1 crystal structure. This model proposes that the IRE1 is activated via two steps. In the first step, BiP dissociation from IRE1 leads to formation of higher order oligomers (called cluster). In the second step, direct interaction of unfolded proteins with IRE1 stabilizes the cytosolic domains of clustered IRE1 molecules and thus causes IRE1 activation. (C) A direct recognition model outlines recent studies in yeast. Three subpopulations of IRE1p co-exist within the cell: an inactive pool in equilibrium with an active unfolded protein-bound pool. The latter is sequestered by BiP binding, designated the third inactive set. In this model, BiP binding to or release from IRE1p does not activate the UPR, but it may serve as a buffer and a timer to adjust the sensitivity and dynamics of IRE1p activity. In turn, the unfolded protein binding to IRE1 is the single step of its activation.
Figure 3
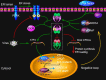
PERK signaling under virus attack. Upon ER stress such as virus infection, protein kinase PERK oligomerizes in the ER membrane and is activated via _trans_-autophosphorylation. The activated PERK phosphorylates a subunit of eIF2, which inhibits the exchange factor eIF2B from recycling eIF2 to its active GTP-bound form. In addition, dsRNA-activated protein kinase R (PKR) can also activate this pathway independently of PERK. The resulting reduced activities of eIF2B and the eIF2 complex account for all of the important consequences of PERK activity, such as translation inhibition of most mRNAs, which reduces protein synthesis and lowers ER loading. However, some mRNA such as ATF4 gains a selective advantage for translation via phosphorylated eIF2. ATF4 in turn contributes to the transcriptional activation of CHOP, XBP1, GADD34, and other genes involved oxidative stress and cell death. GADD34 is a regulatory subunit of protein phosphatase (PP) 1 that dephosphorylates eIF2α and recovers the activity of eIF2, constituting a negative feedback loop for regulation of PERK signaling. A constitutive phosphatase CreP also promotes eIF2 dephosphorylation. Viruses such as CMV may directly exploit the negative loop to terminate the PERK signaling pathway, via increasing the expression of ATF4, because the prolonged closure of protein synthesis is harmful to virus infection. Some viruses, such as HSV1 and ASFV, may produce a viral factor, which is homologous to host GADD34, to restore the activity of eIF2 along with PP1. Other viruses such as HCV may encode a viral protein that binds to PERK as a pseudosubstrate and thus, inhibits PERK activation. Finally, viruses such as LCMV may selectively activate the branches of the UPR to favor their replication. At present, no PERK-like pathway has been found in plants.
Figure 4
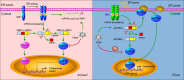
IRE1 signaling and virus infection in animals and plants. In animals, IRE1 oligomerizes in the plane of the ER membrane in stressed cells, leading to _trans_-autophosphorylation and activation. Activated IRE1 mediates the sequence-specific cleavage of the XBP1 mRNA in higher eukaryotes, deleting a small RNA fragment (intron) and finally producing a spliced mRNA (XBP1s) with a frame shift in the coding sequence. Spliced XBP1s encodes a potent transcriptional activator (XBP1s), whereas the unspliced XBP1 mRNA (XBP1u) encodes an inhibitor of the UPR (XBP1u). In mammals, it seems that XBP1s regulates a subset of UPR genes that promote ERAD of misfolded proteins and refold proteins. In cultured Drosophila melanogaster cells, activated IRE1 can promote the cleavage of mRNAs, including XBP1 mRNA, leading to their degradation. This reduces the load on the stressed ER and might facilitate reprogramming of the ER-associated protein synthesis and translocation machinery. In cells infected by viruses such as HCV, the IRE1 pathway is manipulated by the virus via repressing the transcriptional activity of XBP1s. In addition, some viruses might also promote the IRE1-dependent mRNA decay as a means to manipulate the IRE1 pathway. In plants, IRE1 homologs were detected in the genomes of Arabidopsis and rice a decade ago. However, the target of IRE1 was not identified until 2011. The mRNA of transcriptional factor bZIP60 is the substrate of IRE1 in plants. Similar to XBP1 in animals, unspliced bZIP60 (bZIP60u) is processed by activated IRE1. The protein product (bZIP60s) translated from the spliced bZIP60 (bZIPs) is translocated into the nucleus to activate the expression of UPR genes such as chaperones. Different from XBP1u, plant bZIP60u protein, translated from bZIP60u mRNA, is retained in the ER membrane. Sensing unfolded proteins in the ER lumen, bZIP60u undergoes a proteolytic processing, releasing bZIP60s. A recent study has shown that the expression of bZIP60 was increased by PVX infection. However, the roles of the UPR pathway in virus infection have only begun to be investigated in plants. Critical unanswered questions need to be addressed in the future, such as whether viruses modulate the IRE1 pathway via inhibiting the transcriptional activity of bZIP60s (indicated by “?”).
Figure 5
ATF6 and bZIP17/bZIP28 pathways. In unstressed cells, ATF6 in animals and bZIP17/bZIP28 in plants reside in the ER membrane. They are delivered to the Golgi apparatus in an unknown mechanism upon sensing ER stress. In the Golgi apparatus, these proteins are subject to cleavage twice, first by the lumenal S1P and then the intra-membrane S2P, to release the cytosolic effector portions of the proteins (ATF6f). ATF6f then enters into the nucleus and probably activates a subset of UPR target genes, although these remain to be characterized. Some viruses such as ASFV have been shown to selectively activate the ATF6 pathway for their replication in animals. In plants, the cleaved N terminal portions of bZIP17 and bZIP28 also move into the nucleus and activate UPR genes. In plants, the functional roles of IRE1-bZIP17/bZIP28 in virus infection (indicated by “?”) have yet to be elucidated.
Similar articles
- The Human Cytomegalovirus Endoplasmic Reticulum-Resident Glycoprotein UL148 Activates the Unfolded Protein Response.
Siddiquey MNA, Zhang H, Nguyen CC, Domma AJ, Kamil JP. Siddiquey MNA, et al. J Virol. 2018 Sep 26;92(20):e00896-18. doi: 10.1128/JVI.00896-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30045994 Free PMC article. - Protein-rich foods, sea foods, and gut microbiota amplify immune responses in chronic diseases and cancers - Targeting PERK as a novel therapeutic strategy for chronic inflammatory diseases, neurodegenerative disorders, and cancer.
Saaoud F, Lu Y, Xu K, Shao Y, Praticò D, Vazquez-Padron RI, Wang H, Yang X. Saaoud F, et al. Pharmacol Ther. 2024 Mar;255:108604. doi: 10.1016/j.pharmthera.2024.108604. Epub 2024 Feb 13. Pharmacol Ther. 2024. PMID: 38360205 Review. - The PERK Arm of the Unfolded Protein Response Negatively Regulates Transmissible Gastroenteritis Virus Replication by Suppressing Protein Translation and Promoting Type I Interferon Production.
Xue M, Fu F, Ma Y, Zhang X, Li L, Feng L, Liu P. Xue M, et al. J Virol. 2018 Jul 17;92(15):e00431-18. doi: 10.1128/JVI.00431-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29769338 Free PMC article. - A small molecule UPR modulator for diabetes identified by high throughput screening.
Marrocco V, Tran T, Zhu S, Choi SH, Gamo AM, Li S, Fu Q, Cunado MD, Roland J, Hull M, Nguyen-Tran V, Joseph S, Chatterjee AK, Rogers N, Tremblay MS, Shen W. Marrocco V, et al. Acta Pharm Sin B. 2021 Dec;11(12):3983-3993. doi: 10.1016/j.apsb.2021.05.018. Epub 2021 Jun 16. Acta Pharm Sin B. 2021. PMID: 35024320 Free PMC article. - Unfolded Protein Response Signaling in Liver Disorders: A 2023 Updated Review.
Shreya S, Grosset CF, Jain BP. Shreya S, et al. Int J Mol Sci. 2023 Sep 14;24(18):14066. doi: 10.3390/ijms241814066. Int J Mol Sci. 2023. PMID: 37762367 Free PMC article. Review.
Cited by
- Respiratory Syncytial Virus and Cellular Stress Responses: Impact on Replication and Physiopathology.
Cervantes-Ortiz SL, Zamorano Cuervo N, Grandvaux N. Cervantes-Ortiz SL, et al. Viruses. 2016 May 12;8(5):124. doi: 10.3390/v8050124. Viruses. 2016. PMID: 27187445 Free PMC article. Review. - Interplay between Inflammation and Cellular Stress Triggered by Flaviviridae Viruses.
Valadão AL, Aguiar RS, de Arruda LB. Valadão AL, et al. Front Microbiol. 2016 Aug 25;7:1233. doi: 10.3389/fmicb.2016.01233. eCollection 2016. Front Microbiol. 2016. PMID: 27610098 Free PMC article. Review. - Biogenesis and Breakdown of Lipid Droplets in Pathological Conditions.
Fader Kaiser CM, Romano PS, Vanrell MC, Pocognoni CA, Jacob J, Caruso B, Delgui LR. Fader Kaiser CM, et al. Front Cell Dev Biol. 2022 Feb 7;9:826248. doi: 10.3389/fcell.2021.826248. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35198567 Free PMC article. Review. - Endoplasmic reticulum retention motif fused to recombinant anti-cancer monoclonal antibody (mAb) CO17-1A affects mAb expression and plant stress response.
Song I, Kang Y, Lee YK, Myung SC, Ko K. Song I, et al. PLoS One. 2018 Sep 24;13(9):e0198978. doi: 10.1371/journal.pone.0198978. eCollection 2018. PLoS One. 2018. PMID: 30248125 Free PMC article. - Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation.
Wang Y, Li JR, Sun MX, Ni B, Huan C, Huang L, Li C, Fan HJ, Ren XF, Mao X. Wang Y, et al. Antiviral Res. 2014 Jun;106:33-41. doi: 10.1016/j.antiviral.2014.03.007. Epub 2014 Mar 25. Antiviral Res. 2014. PMID: 24681123 Free PMC article.
References
- Baltzis D., Qu L. K., Papadopoulou S., Blais J. D., Bell J. C., Sonenberg N., et al. (2004). Resistance to vesicular stomatitis virus infection requires a functional cross talk between the eukaryotic translation initiation factor 2α kinases PERK and PKR. J. Virol. 78, 12747–12761 10.1128/JVI.78.23.12747-12761.2004 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials