Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy - PubMed (original) (raw)
Review
Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy
Chia-Chen Liu et al. Nat Rev Neurol. 2013 Feb.
Erratum in
- Nat Rev Neurol. 2013. doi: 10.1038/nmeurol.2013.32. Liu, Chia-Chan [corrected to Liu, Chia-Chen]
Abstract
Apolipoprotein E (Apo-E) is a major cholesterol carrier that supports lipid transport and injury repair in the brain. APOE polymorphic alleles are the main genetic determinants of Alzheimer disease (AD) risk: individuals carrying the ε4 allele are at increased risk of AD compared with those carrying the more common ε3 allele, whereas the ε2 allele decreases risk. Presence of the APOE ε4 allele is also associated with increased risk of cerebral amyloid angiopathy and age-related cognitive decline during normal ageing. Apo-E-lipoproteins bind to several cell-surface receptors to deliver lipids, and also to hydrophobic amyloid-β (Aβ) peptide, which is thought to initiate toxic events that lead to synaptic dysfunction and neurodegeneration in AD. Apo-E isoforms differentially regulate Aβ aggregation and clearance in the brain, and have distinct functions in regulating brain lipid transport, glucose metabolism, neuronal signalling, neuroinflammation, and mitochondrial function. In this Review, we describe current knowledge on Apo-E in the CNS, with a particular emphasis on the clinical and pathological features associated with carriers of different Apo-E isoforms. We also discuss Aβ-dependent and Aβ-independent mechanisms that link Apo-E4 status with AD risk, and consider how to design effective strategies for AD therapy by targeting Apo-E.
Figures
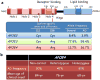
Figure 1. APOE ε4 is a major genetic risk factor for Alzheimer disease
(a) The ApoE2, E3, and E4 isoforms, which are encoded by the ε2, ε3 and ε4 alleles of the APOE gene, respectively, differ from one another at amino acid residues 112 and/or 158 (red circles). ApoE has two structural domains: the N-terminal domain, which contains the receptor-binding region (residues 136–150), and the C-terminal domain, which contains the lipid-binding region (residues 244–272); the two domains are joined by a hinge region. A meta-analysis demonstrated a significant association between the ε4 allele of APOE and AD. (b) APOE ε4 increases the risk of AD and lowers the age of disease onset in a gene-dose-dependent manner., Abbreviations: AD, Alzheimer disease; ApoE, Apolipoprotein E.
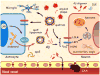
Figure 2. Apolipoprotein E and amyloid-β metabolism in the brain
The main Aβ clearance pathways include receptor-mediated uptake by neurons and glia, drainage into interstitial fluid or through the BBB, and proteolytic degradation by IDE and neprilysin. Impaired clearance of Aβ can cause Aβ accumulation in brain parenchyma, leading to formation of neurotoxic Aβ oligomers and amyloid plaques. Aβ accumulation in the perivascular region leads to CAA, which disrupts blood vessel function. ApoE is primarily synthesized by astrocytes and microglia, and is lipidated by the ABCA1 transporter to form lipoprotein particles. Lipidated ApoE binds to soluble Aβ and facilitates Aβ uptake through cell surface receptors, including LRP1, LDLR, and HSPG, in a manner that probably depends on ApoE isoform and its level of lipidation. ApoE facilitates binding and internalization of soluble Aβ by glial cells, disrupts Aβ clearance at the BBB in an isoform-dependent manner (ApoE4 > ApoE3 > ApoE2) and influences CAA pathogenesis. Abbreviations: Aβ, amyloid-β; ABCA1, ATP-binding cassette A1; BBB, blood–brain barrier; CAA, cerebral amyloid angiopathy; HSPG, heparan sulphate proteoglycan; IDE, insulin-degrading enzyme; LDLR, low-density lipoprotein receptor; LRP1, low-density lipoprotein receptor-related protein 1; LXR, liver X receptor.
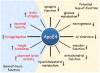
Figure 3. The role of Apolipoprotein E4 in Alzheimer disease pathogenesis
ApoE4 confers toxic gain of function, loss of neuroprotective function or both in the pathogenesis of Alzheimer disease. Key functional differences between ApoE4 and ApoE3 are illustrated. Abbreviations: Aβ, amyloid-β; ApoE, Apolipoprotein E.
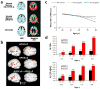
Figure 4. Abnormal brain function and enhanced neuropathology and memory decline in cognitively normal APOE ε4 carriers
(a) 18F-fluorodeoxyglucose PET images show that cognitively normal APOE ε4 carriers have lower glucose metabolism than do noncarriers. (b) APOE ε4 carriers exhibit a greater increase in functional MRI signal in brain regions associated with task performance, and show increases in additional regions compared with APOE ε3 carriers. (c) Age-related memory decline occurs more rapidly in APOE ε4 carriers than noncarriers, starting from age 55–60 years. (d) APOE ε4 carriers show increased cerebral Aβ deposition which persists in greater frequencies with age compared with noncarriers. Increased PiB binding and reduced CSF Aβ42 levels reflect cerebral amyloid deposition. Abbreviations: Aβ, amyloid-β; APOE, apolipoprotein E; CSF, cerebrospinal fluid; PiB, Pittsburg compund B. Part a, is modified, with permission from the National Academy of Science, USA © Small, G. W. et al. Proc. Natl Acad. Sci. USA 97, 6037–6042 (2000). Part b, is modified, with permission from the Massachusetts Medical Society © Bookheimer, S. Y. et al. N. Engl. J. Med. 343, 450–456 (2000). Part c, is modified, with permission from the Massachusetts Medical Society © Caselli et al. N. Engl. J. Med. 361, 255–263 (2009). Part d is modified, with permission, from John Wiley and Sons © Morris, J. C. et al. Ann. Neurol. 67, 122–131 (2010).
Similar articles
- [Role of apolipoprotein E in the molecular pathomechanism of Alzheimer disease].
Michikawa M. Michikawa M. Nihon Shinkei Seishin Yakurigaku Zasshi. 2014 Feb;34(1):5-9. Nihon Shinkei Seishin Yakurigaku Zasshi. 2014. PMID: 25069266 Review. Japanese. - Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease.
Holtzman DM, Herz J, Bu G. Holtzman DM, et al. Cold Spring Harb Perspect Med. 2012 Mar;2(3):a006312. doi: 10.1101/cshperspect.a006312. Cold Spring Harb Perspect Med. 2012. PMID: 22393530 Free PMC article. Review. - Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies.
Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Yamazaki Y, et al. Nat Rev Neurol. 2019 Sep;15(9):501-518. doi: 10.1038/s41582-019-0228-7. Epub 2019 Jul 31. Nat Rev Neurol. 2019. PMID: 31367008 Free PMC article. Review. - Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease.
Lautner R, Palmqvist S, Mattsson N, Andreasson U, Wallin A, Pålsson E, Jakobsson J, Herukka SK, Owenius R, Olsson B, Hampel H, Rujescu D, Ewers M, Landén M, Minthon L, Blennow K, Zetterberg H, Hansson O; Alzheimer’s Disease Neuroimaging Initiative. Lautner R, et al. JAMA Psychiatry. 2014 Oct;71(10):1183-91. doi: 10.1001/jamapsychiatry.2014.1060. JAMA Psychiatry. 2014. PMID: 25162367 - APOE-amyloid interaction: Therapeutic targets.
Wisniewski T, Drummond E. Wisniewski T, et al. Neurobiol Dis. 2020 May;138:104784. doi: 10.1016/j.nbd.2020.104784. Epub 2020 Feb 4. Neurobiol Dis. 2020. PMID: 32027932 Free PMC article. Review.
Cited by
- Ionising radiation exposure-induced regulation of selected biomarkers and their impact in cancer and treatment.
Mzizi Y, Mbambara S, Moetlhoa B, Mahapane J, Mdanda S, Sathekge M, Kgatle M. Mzizi Y, et al. Front Nucl Med. 2024 Oct 21;4:1469897. doi: 10.3389/fnume.2024.1469897. eCollection 2024. Front Nucl Med. 2024. PMID: 39498386 Free PMC article. Review. - Computational Modeling of Lipid Metabolism in Yeast.
Schützhold V, Hahn J, Tummler K, Klipp E. Schützhold V, et al. Front Mol Biosci. 2016 Sep 27;3:57. doi: 10.3389/fmolb.2016.00057. eCollection 2016. Front Mol Biosci. 2016. PMID: 27730126 Free PMC article. - The Importance of Complement-Mediated Immune Signaling in Alzheimer's Disease Pathogenesis.
Batista AF, Khan KA, Papavergi MT, Lemere CA. Batista AF, et al. Int J Mol Sci. 2024 Jan 9;25(2):817. doi: 10.3390/ijms25020817. Int J Mol Sci. 2024. PMID: 38255891 Free PMC article. Review. - Quantitative and Kinetic Proteomics Reveal ApoE Isoform-dependent Proteostasis Adaptations in Mouse Brain.
Zuniga NR, Earls NE, Denos AEA, Elison JM, Jones BS, Smith EG, Moran NG, Brown KL, Romero GM, Hyer CD, Wagstaff KB, Almughamsi HM, Transtrum MK, Price JC. Zuniga NR, et al. bioRxiv [Preprint]. 2024 Aug 14:2024.08.13.607719. doi: 10.1101/2024.08.13.607719. bioRxiv. 2024. PMID: 39185235 Free PMC article. Updated. Preprint. - Second to fourth digit ratio (2D:4D) is associated with dementia in women.
Jiang J, Young K, Pike CJ. Jiang J, et al. Early Hum Dev. 2020 Oct;149:105152. doi: 10.1016/j.earlhumdev.2020.105152. Epub 2020 Aug 6. Early Hum Dev. 2020. PMID: 32781308 Free PMC article.
References
- Alzheimer’s Association. 2012 Alzheimer’s disease facts and figures. Alzheimer’s dement. 2012;8:131–168. - PubMed
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. - PubMed
- Roberson ED, et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG035355/AG/NIA NIH HHS/United States
- R01 AG038710/AG/NIA NIH HHS/United States
- R01 AG021173/AG/NIA NIH HHS/United States
- P01 NS074969/NS/NINDS NIH HHS/United States
- R01 AG044420/AG/NIA NIH HHS/United States
- RF1 AG046205/AG/NIA NIH HHS/United States
- R01 AG031784/AG/NIA NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous