AMP-activated protein kinase is required for exercise-induced peroxisome proliferator-activated receptor co-activator 1 translocation to subsarcolemmal mitochondria in skeletal muscle - PubMed (original) (raw)
AMP-activated protein kinase is required for exercise-induced peroxisome proliferator-activated receptor co-activator 1 translocation to subsarcolemmal mitochondria in skeletal muscle
Brennan K Smith et al. J Physiol. 2013.
Abstract
In skeletal muscle, mitochondria exist as two subcellular populations known as subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria. SS mitochondria preferentially respond to exercise training, suggesting divergent transcriptional control of the mitochondrial genomes. The transcriptional co-activator peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) and mitochondrial transcription factor A (Tfam) have been implicated in the direct regulation of the mitochondrial genome in mice, although SS and IMF differences may exist, and the potential signalling events regulating the mitochondrial content of these proteins have not been elucidated. Therefore, we examined the potential for PGC-1α and Tfam to translocate to SS and IMF mitochondria in human subjects, and performed experiments in rodents to identify signalling mechanisms regulating these translocation events. Acute exercise in humans and rats increased PGC-1α content in SS but not IMF mitochondria. Acute exposure to 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside in rats recapitulated the exercise effect of increased PGC-1α protein within SS mitochondria only, suggesting that AMP-activated protein kinase (AMPK) signalling is involved. In addition, rendering AMPK inactive (AMPK kinase dead mice) prevented exercise-induced PGC-1α translocation to SS mitochondria, further suggesting that AMPK plays an integral role in these translocation events. In contrast to the conserved PGC-1α translocation to SS mitochondria across species (humans, rats and mice), acute exercise only increased mitochondrial Tfam in rats. Nevertheless, in rat resting muscle PGC-1α and Tfam co-immunoprecipate with α-tubulin, suggesting a common cytosolic localization. These data suggest that exercise causes translocation of PGC-1α preferentially to SS mitochondria in an AMPK-dependent manner.
Figures
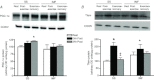
Figure 1. Mitochondrial isolation cleanliness checks
A, to ensure that our mitochondrial isolation procedures yielded sample devoid of nuclear contamination we loaded 5, 10, 20 30 μg of isolated mitochondrial protein (subsarcolemmal (SS) and intermyofibrillar (IMF)) and probed for H2B (nuclear protein) and COXIV (mitochondrial protein). This check was performed on the same membrane and indicates that our isolation procedure yields highly purified mitochondria. B, to confirm that PGC-1α exists within both the SS and the IMF fraction, 10 μg of mitochondrial protein was loaded and detected in concert with other mitochondrial proteins and the absence of non-mitochondrial proteins. Thirty micrograms of muscle homogenate protein was loaded.
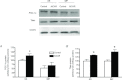
Figure 2. In human skeletal muscle, exercise promotes PGC-1α translocation to subsarcolemmal mitochondria
A, representative control blots to ensure purified mitochondrial samples. B, following an acute bout of exercise at 60% for 120 min, PGC-1α is increased in subsarcolemmal (SS) mitochondria 3 h post-exercise. C, in contrast, Tfam is not increased in either mitochondrial population; n = 8 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
for 120 min, PGC-1α is increased in subsarcolemmal (SS) mitochondria 3 h post-exercise. C, in contrast, Tfam is not increased in either mitochondrial population; n = 8 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
Figure 3. Changes in rat skeletal muscle PGC-1α and Tfam subcellular localization following exhaustive exercise
A, following 3 h of recovery PGC-1α is increased in subsarcolemmal (SS) mitochondria. B, immediately post-exercise, Tfam is transiently increased in both the SS and the intermyofibrillar (IMF) mitochondria; n = 6 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions.
Figure 4. Acute 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside (AICAR) treatment increases PGC-1α and Tfam in a mitochondrial population-specific manner
Rat skeletal muscle was harvested 60 min after an intraperitoneal injection (1 mg g body weight−1) of AICAR. A, acute AICAR treatment increases PGC-1α in SS mitochondria. B, acute AICAR treatment increases Tfam in both SS and intermyofibrillar (IMF) mitochondria; n = 4 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions.
Figure 5. Differences in exercise-induced PGC-1α translocation between AMPK KD and WT mice
A, following exhaustive exercise, PGC-1α is increased in the subsarcolemmal (SS) mitochondria of WT mice immediately post and 3 h post exercise. B, in contrast, in AMPK KD mice, exercise did not induce PGC-1α translocation to either mitochondrial population. C and D, Tfam is not increased in either mitochondrial population following exhaustive exercise; n = 4 for all independent experiments. Data are expressed as mean ± SEM. *P < 0.05, significantly different from rest. Ten micrograms of mitochondrial protein was loaded for all conditions. IMF, intermyofibrillar mitochondria.
Figure 6. PGC-1α and Tfam co-localize with α-tubulin in the cytosol
PGC-1α and Tfam co-immunoprecipitate (IP) with each other and with α-tubulin. In contrast, lactate dehydrogenase (LDH), cytochrome c oxidase complex IV (COXIV) and pyruvate dehydrogenase (PDHE1α) do not co-immunoprecipitate with Tfam. Gels were cut simultaneously; n = 4 for all independent experiments. Two independent representative blots shown. C, control elutant wash-through.
Similar articles
- Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria.
Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Benton CR, et al. J Biol Chem. 2008 Feb 15;283(7):4228-40. doi: 10.1074/jbc.M704332200. Epub 2007 Dec 12. J Biol Chem. 2008. PMID: 18079123 - Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α.
Kang C, Chung E, Diffee G, Ji LL. Kang C, et al. Exp Gerontol. 2013 Nov;48(11):1343-50. doi: 10.1016/j.exger.2013.08.004. Epub 2013 Aug 30. Exp Gerontol. 2013. PMID: 23994518 - Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle.
Gurd BJ, Yoshida Y, McFarlan JT, Holloway GP, Moyes CD, Heigenhauser GJ, Spriet L, Bonen A. Gurd BJ, et al. Am J Physiol Regul Integr Comp Physiol. 2011 Jul;301(1):R67-75. doi: 10.1152/ajpregu.00417.2010. Epub 2011 May 4. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21543634 - Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.
Gurd BJ. Gurd BJ. Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review. - PGC-1alpha-induced improvements in skeletal muscle metabolism and insulin sensitivity.
Bonen A. Bonen A. Appl Physiol Nutr Metab. 2009 Jun;34(3):307-14. doi: 10.1139/H09-008. Appl Physiol Nutr Metab. 2009. PMID: 19448691 Review.
Cited by
- The Implication of PGC-1α on Fatty Acid Transport across Plasma and Mitochondrial Membranes in the Insulin Sensitive Tissues.
Supruniuk E, Mikłosz A, Chabowski A. Supruniuk E, et al. Front Physiol. 2017 Nov 15;8:923. doi: 10.3389/fphys.2017.00923. eCollection 2017. Front Physiol. 2017. PMID: 29187824 Free PMC article. Review. - How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus.
Bolijn S, Lucassen PJ. Bolijn S, et al. Brain Plast. 2015 Oct 9;1(1):5-27. doi: 10.3233/BPL-150020. Brain Plast. 2015. PMID: 29765833 Free PMC article. Review. - Transcriptional modulation of mitochondria biogenesis pathway at and above critical speed in mice.
Mille-Hamard L, Breuneval C, Rousseau AS, Grimaldi P, Billat VL. Mille-Hamard L, et al. Mol Cell Biochem. 2015 Jul;405(1-2):223-32. doi: 10.1007/s11010-015-2413-3. Epub 2015 Apr 26. Mol Cell Biochem. 2015. PMID: 25912548 - Metformin Protects Rat Skeletal Muscle from Physical Exercise-Induced Injury.
Abbadessa G, Maniscalco E, Grasso L, Popara J, Di Scipio F, Franco F, Mancardi D, Pigozzi F, Borrione P, Berta GN, Racca S. Abbadessa G, et al. Biomedicines. 2023 Aug 22;11(9):2334. doi: 10.3390/biomedicines11092334. Biomedicines. 2023. PMID: 37760776 Free PMC article. - The Molecular Signature of High-intensity Training in the Human Body.
Wahl P, Bloch W, Proschinger S. Wahl P, et al. Int J Sports Med. 2022 Mar;43(3):195-205. doi: 10.1055/a-1551-9294. Epub 2021 Oct 12. Int J Sports Med. 2022. PMID: 34265857 Free PMC article. Review.
References
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway 1. J Biol Chem. 2005;280:19587–19593. - PubMed
- Benton CR, Nickerson JG, Lally J, Han XX, Holloway GP, Glatz JF, Luiken JJ, Graham TE, Heikkila JJ, Bonen A. Modest PGC-1α overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J Biol Chem. 2008b;283:4228–4240. - PubMed
- Bizeau ME, Willis WT, Hazel JR. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J Appl Physiol. 1998;85:1279–1284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases