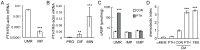Amphiregulin-EGFR signaling mediates the migration of bone marrow mesenchymal progenitors toward PTH-stimulated osteoblasts and osteocytes - PubMed (original) (raw)
Amphiregulin-EGFR signaling mediates the migration of bone marrow mesenchymal progenitors toward PTH-stimulated osteoblasts and osteocytes
Ji Zhu et al. PLoS One. 2012.
Abstract
Intermittent administration of parathyroid hormone (PTH) dramatically increases bone mass and currently is one of the most effective treatments for osteoporosis. However, the detailed mechanisms are still largely unknown. Here we demonstrate that conditioned media from PTH-treated osteoblastic and osteocytic cells contain soluble chemotactic factors for bone marrow mesenchymal progenitors, which express a low amount of PTH receptor (PTH1R) and do not respond to PTH stimulation by increasing cAMP production or migrating toward PTH alone. Conditioned media from PTH-treated osteoblasts elevated phosphorylated Akt and p38MAPK amounts in mesenchymal progenitors and inhibition of these pathways blocked the migration of these progenitors toward conditioned media. Our previous and current studies revealed that PTH stimulates the expression of amphiregulin, an epidermal growth factor (EGF)-like ligand that signals through the EGF receptor (EGFR), in both osteoblasts and osteocytes. Interestingly, conditioned media from PTH-treated osteoblasts increased EGFR phosphorylation in mesenchymal progenitors. Using several different approaches, including inhibitor, neutralizing antibody, and siRNA, we demonstrate that PTH increases the release of amphiregulin from osteoblastic cells, which acts on the EGFRs expressed on mesenchymal progenitors to stimulate the Akt and p38MAPK pathways and subsequently promote their migration in vitro. Furthermore, inactivation of EGFR signaling specifically in osteoprogenitors/osteoblasts attenuated the anabolic actions of PTH on bone formation. Taken together, these results suggest a novel mechanism for the therapeutic effect of PTH on osteoporosis and an important role of EGFR signaling in mediating PTH's anabolic actions on bone.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
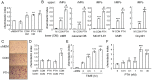
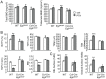
Figure 1. Conditioned media from PTH-treated osteoblastic cells contain chemotactic factors for bone marrow mesenchymal progenitors.
(A) Migration of freshly flushed rat bone marrow cells toward PTH and conditioned media from UMR 106-01 cells. Bone marrow cells flushed from rat long bones were immediately seeded in the upper chamber of transwell plates. The bottom wells were loaded with media alone (αMEM), media containing 10 nM PTH (PTH), or conditioned media (CM) collected from UMR106-01 cells that had been treated with control (CON) or 10 nM PTH (PTH) for 4 hr. The number of cells that migrated to the bottom wells was counted 24 hr later. αMEM containing 5% FBS (FBS) was used as a positive control for cell migration. *: p<0.05; **: p<0.01 vs. αMEM. (B) Conditioned media from various PTH-treated osteoblastic and osteocytic cells stimulated the migration of either human or rat mesenchymal progenitors (MP) in the Boyden chamber assay. The cells seeded in the upper wells are depicted at the top and conditioned media loaded in the lower wells are shown at the bottom. M: αMEM. ***: p<0.001 vs. αMEM; &: p<0.01; #: p<0.001 vs. CON. (C) Microscopic images of the mesenchymal progenitors that migrated to the lower sides of filters in the assay depicted in B. (D) The migration of mesenchymal progenitors stimulated by conditioned media from PTH-treated osteoblasts is not chemokinesis. Mesenchymal progenitors were suspended in conditioned media harvested from either control or PTH-treated UMR 106-01 cells and seeded in the upper chambers. The lower wells were filled with conditioned media, resulting in a total of 4 combination types. **: p<0.01 vs. CON/CON. (E) Time course of the release of chemotactic factor(s) from osteoblasts after PTH treatment. UMR 106-01 cells were treated with PTH and conditioned media were harvested at indicated time points and loaded in the lower wells for chemotaxis assays. ***: p<0.001 vs. time 0 hr. (F) The dosage effects of PTH on chemotactic factor(s) release from osteoblasts. UMR 106-01 cells were treated with different doses of PTH for 4 hr and then conditioned media were harvested for chemotaxis assays. *: p<0.05; **: p = 0.06; ***: p<0.001 vs. 0 nM.
Figure 2. PTH itself is not a chemotactic factor for mesenchymal progenitors.
(A) qRT-PCR quantification of mRNA levels of PTH1R in UMR106-01 cells and rat mesenchymal progenitors in culture. ***: p<0.001 vs. UMR. (B) The expression of PTH1R in rat calvarial osteoblasts increases dramatically during their osteogenic differentiation as measured by qRT-PCR. PRO: proliferation stage; DIF: differentiation stage; MIN: mineralization stage. **: p<0.01; ***: p<0.001 vs. PRO. (C) PTH stimulates cAMP production in UMR 106-01 cells but not mesenchymal progenitors. ***: p<0.001 vs. con. (D) PTH alone does not stimulate the migration of mesenchymal progenitors. Chemotaxis assays were performed with lower wells filled with αMEM, αMEM containing 10 nM PTH, conditioned media from control- and PTH-treated UMR 106-01 cells, and αMEM containing 5% FBS. ***: p<0.001 vs. CON.
Figure 3. PI3K/Akt and p38MAPK pathways are required for the migration of mesenchymal progenitors toward conditioned media from PTH-treated osteoblastic cells.
(A) Chemotaxis assays were performed with mesenchymal progenitors and conditioned media from either control- or PTH-treated UMR 106-01 cells in the presence or absence of pathway-specific inhibitors. D: DMSO; U: U0126 (20 µM); WT: wortmannin (3 µM); SB: SB202190 (20 µM). Inhibitors were added to both upper and bottom chambers. ***: p<0.001 vs. CON CM D; #: p<0.001 vs. PTH CM D. (B) Conditioned media from PTH-treated UMR 106-01 cells stimulated the phosphorylation of Akt and p38MAPK in MSCs. (C) PTH alone did not activate Akt and p38MAPK pathways in mesenchymal progenitors.
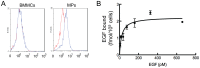
Figure 4. Mesenchymal progenitors express EGFR.
(A) Flow cytometry analyses demonstrate that cultured mesenchymal progenitors (MPs) but not freshly isolated bone marrow mononuclear cells (BMMCs) express EGFR surface antigen. Blue curve: EGFR antibody; red curve: isotype control. (B) Saturation curve of binding of 125I-EGF to mesenchymal progenitors.
Figure 5. EGFR signaling mediates the chemotactic effect of PTH on mesenchymal progenitors.
(A) Conditioned media from PTH-treated UMR 106-01 cells increased the phosphorylation of EGFR in mesenchymal progenitors. In this experiment, mesenchymal progenitors were treated with conditioned media for 5 min and then lysed for Western blot. (B) The enhanced phosphorylation of Akt and p38MAPK in mesenchymal progenitors by conditioned media from PTH-treated UMR106-01 cells is dependent on the EGFR pathway. Mesenchymal progenitors were pre-incubated with either DMSO or gefitinib (10 µM, GEF) for 30 min followed by addition of conditioned media to the culture. Cell lysates were collected 5 min later for Western blot analyses. (C) The EGFR inhibitor PD153035 (10 µM, PD) was added to both the upper and lower wells of the chemotaxis assay and partially blocked the PTH-induced chemotactic activity of conditioned media from UMR 106-01 cells. ***: p<0.001 vs. DMSO CON; &: p<0.01 vs. DMSO PTH. (D) An EGFR neutralizing antibody (4 µg/ml) was mixed with mesenchymal progenitors before the chemotaxis assay and suppressed the migration of mesenchymal progenitors towards conditioned media from PTH-treated UMR 106-01 cells. IgG: isotype control. **: p<0.01; ***: p<0.001 vs. CON; $: p<0.05; #: p<0.001 vs. PTH. (E) qRT-PCR demonstrates the knockdown of EGFR mRNA levels in mesenchymal progenitors by siRNAs. ***: p<0.001 vs. MOCK. (F) Immunoblotting reveals that the EGFR protein level was dramatically decreased in mesenchymal progenitors transfected with siRNAs for EGFR. (G) Blocking of EGFR expression in mesenchymal progenitors by siRNAs abolished the chemotactic migration of these cells toward conditioned media from PTH-treated UMR 106-01 cells. ***: p<0.001 vs. mock CON; #: p<0.001 vs. mock PTH.
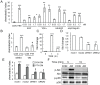
Figure 6. PTH stimulates the release of amphiregulin from osteoblasts to promote mesenchymal progenitor migration.
(A) EGF-like ligands are chemotactic factors for mesenchymal progenitors. αMEM containing various amounts of EGF-like ligands was added in the lower wells of chemotaxis assays using rat mesenchymal progenitors. αMEM containing 5% FBS was used as positive control. *: p<0.05; **: p<0.01; ***: p<0.001 vs. CON. (B) Addition of GM6001 (10 µM, GM) in the chemotaxis assay blocked the migration of mesenchymal progenitors toward conditioned media from PTH-treated UMR 106-01 cells. ***: p<0.001 vs. CON DMSO; #: p<0.001 vs. PTH DMSO. (C) qRT-PCR shows that PTH (10 nM) induced the expression of amphiregulin in osteocytic Ocy491 cells at 1 h. (D) qRT-PCR demonstrates the knockdown of amphiregulin mRNA in UMR 106-01 cells after 1 hr of PTH (10 nM) treatment by siRNAs. **: p<0.01 vs. mock1. (E) Chemotaxis assays reveal that PTH did not stimulate the release of chemotactic factor(s) from UMR106-01 cells transfected with siRNAs for amphiregulin. ***: p<0.001 vs. CON CM; #; p<0.001 vs PTH CM mock1. (F) Amphiregulin (AR) stimulated Akt and p38MAPK phosphorylation in mesenchymal progenitors as shown by immunoblotting.
Figure 7. The anabolic actions of PTH on trabecular bone are attenuated in EGFR-deficient mice.
(A) pQCT measurement of total and trabecular BMDs of the proximal tibiae of vehicle- or PTH-injected Col-Cre EgfrWa5/flox mice, their Wa5 (EgfrWa5/flox) and wild type (WT) siblings. (B) Structural parameters of trabecular bone in the proximal tibiae of vehicle- or PTH-injected Col-Cre EgfrWa5/flox and WT mice. BV/TV: trabecular bone volume/tissue volume; TbTh: trabecular thickness; TbSp: trabecular separation; TbN: trabecular number; TbPf: trabecular pattern factor; SMI: structure model index. (C) Bone histomorphometry revealed that PTH-induced osteoblast formation is blunted in Col-Cre EgfrWa5/flox mice. *: p<0.05; **: p<0.01; ***: p<0.001 PTH vs. veh; &: p<0.05; #: p<0.01 vehicle-treated Col-Cre EgfrWa5/flox vs vehicle-treated WT mice. n = 5–7 mice per group.
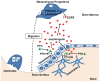
Figure 8. A model for PTH-induced mesenchymal progenitor migration in bone.
Similar articles
- Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation.
Qin L, Tamasi J, Raggatt L, Li X, Feyen JH, Lee DC, Dicicco-Bloom E, Partridge NC. Qin L, et al. J Biol Chem. 2005 Feb 4;280(5):3974-81. doi: 10.1074/jbc.M409807200. Epub 2004 Oct 27. J Biol Chem. 2005. PMID: 15509566 - gp130 in late osteoblasts and osteocytes is required for PTH-induced osteoblast differentiation.
Standal T, Johnson RW, McGregor NE, Poulton IJ, Ho PW, Martin TJ, Sims NA. Standal T, et al. J Endocrinol. 2014 Nov;223(2):181-90. doi: 10.1530/JOE-14-0424. Epub 2014 Sep 16. J Endocrinol. 2014. PMID: 25228504 - Reconstitution of amphiregulin-epidermal growth factor receptor signaling in lung squamous cell carcinomas activates PTHrP gene expression and contributes to cancer-mediated diseases of the bone.
Gilmore JL, Gonterman RM, Menon K, Lorch G, Riese DJ 2nd, Robling A, Foley J. Gilmore JL, et al. Mol Cancer Res. 2009 Oct;7(10):1714-28. doi: 10.1158/1541-7786.MCR-09-0131. Epub 2009 Oct 13. Mol Cancer Res. 2009. PMID: 19825997 Free PMC article. - Does osteocytic SOST suppression mediate PTH bone anabolism?
Kramer I, Keller H, Leupin O, Kneissel M. Kramer I, et al. Trends Endocrinol Metab. 2010 Apr;21(4):237-44. doi: 10.1016/j.tem.2009.12.002. Epub 2010 Jan 13. Trends Endocrinol Metab. 2010. PMID: 20074973 Review. - Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies.
Esbrit P, Alcaraz MJ. Esbrit P, et al. Biochem Pharmacol. 2013 May 15;85(10):1417-23. doi: 10.1016/j.bcp.2013.03.002. Epub 2013 Mar 13. Biochem Pharmacol. 2013. PMID: 23500550 Review.
Cited by
- PTH and the Regulation of Mesenchymal Cells within the Bone Marrow Niche.
Liu H, Liu L, Rosen CJ. Liu H, et al. Cells. 2024 Feb 26;13(5):406. doi: 10.3390/cells13050406. Cells. 2024. PMID: 38474370 Free PMC article. Review. - Small interfering RNAs in the management of human osteoporosis.
Gargano G, Asparago G, Spiezia F, Oliva F, Maffulli N. Gargano G, et al. Br Med Bull. 2023 Dec 11;148(1):58-69. doi: 10.1093/bmb/ldad023. Br Med Bull. 2023. PMID: 37675799 Free PMC article. Review. - Epidermal growth factor signalling pathway in endochondral ossification: an evidence-based narrative review.
Mangiavini L, Peretti GM, Canciani B, Maffulli N. Mangiavini L, et al. Ann Med. 2022 Dec;54(1):37-50. doi: 10.1080/07853890.2021.2015798. Ann Med. 2022. PMID: 34955078 Free PMC article. Review. - Expression of Amphiregulin in Enchondromas and Central Chondrosarcomas.
Losada DM, Ribeiro ALDC, Cintra FF, Mendonça GRA, Etchebehere M, Amstalden EMI. Losada DM, et al. Clinics (Sao Paulo). 2021 Aug 27;76:e2914. doi: 10.6061/clinics/2021/e2914. eCollection 2021. Clinics (Sao Paulo). 2021. PMID: 34468540 Free PMC article. - Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain.
Zou W, Rohatgi N, Brestoff JR, Li Y, Barve RA, Tycksen E, Kim Y, Silva MJ, Teitelbaum SL. Zou W, et al. Cell Metab. 2020 Nov 3;32(5):801-813.e6. doi: 10.1016/j.cmet.2020.09.011. Epub 2020 Oct 6. Cell Metab. 2020. PMID: 33027637 Free PMC article.
References
- Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R (1993) Anabolic actions of parathyroid hormone on bone. Endocr Rev 14: 690–709. - PubMed
- Tam CS, Heersche JN, Murray TM, Parsons JA (1982) Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110: 506–512. - PubMed
- Rouleau MF, Mitchell J, Goltzman D (1988) In vivo distribution of parathyroid hormone receptors in bone: evidence that a predominant osseous target cell is not the mature osteoblast. Endocrinology 123: 187–191. - PubMed
- Fermor B, Skerry TM (1995) PTH/PTHrP receptor expression on osteoblasts and osteocytes but not resorbing bone surfaces in growing rats. J Bone Miner Res 10: 1935–1943. - PubMed
- Qin L, Raggatt LJ, Partridge NC (2004) Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab 15: 60–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R25 CA101871/CA/NCI NIH HHS/United States
- R25 CA101871-07/CA/NCI NIH HHS/United States
- K01-DK071988/DK/NIDDK NIH HHS/United States
- K01 DK071988/DK/NIDDK NIH HHS/United States
- UH2 AR059655/AR/NIAMS NIH HHS/United States
- UH2-AR059655/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous