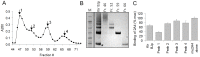AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils - PubMed (original) (raw)
AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils
Jonathan S Wall et al. PLoS One. 2012.
Abstract
The monoclonal antibody 2A4 binds an epitope derived from a cleavage site of serum amyloid protein A (sAA) containing a -Glu-Asp- amino acid pairing. In addition to its reactivity with sAA amyloid deposits, the antibody was also found to bind amyloid fibrils composed of immunoglobulin light chains. The antibody binds to synthetic fibrils and human light chain (AL) amyloid extracts with high affinity even in the presence of soluble light chain proteins. Immunohistochemistry with biotinylated 2A4 demonstrated positive reaction with ALκ and ALλ human amyloid deposits in various organs. Surface plasmon resonance analyses using synthetic AL fibrils as a substrate revealed that 2A4 bound with a K(D) of ∼10 nM. Binding was inhibited in the presence of the -Glu-Asp- containing immunogen peptide. Radiolabeled 2A4 specifically localized with human AL amyloid extracts implanted in mice (amyloidomas) as evidenced by single photon emission (SPECT) imaging. Furthermore, co-localization of the radiolabeled mAb with amyloid was shown in biodistribution and micro-autoradiography studies. Treatment with 2A4 expedited regression of ALκ amyloidomas in mice, likely mediated by the action of macrophages and neutrophils, relative to animals that received a control antibody. These data indicate that the 2A4 mAb might be of interest for potential imaging and immunotherapy in patients with AL amyloidosis.
Conflict of interest statement
Competing Interests: RB, PS, and DS are employees of Neotope Biosciences/Elan. JSW and SJK receive royalties from a patent associated with the 2A4 mAb (US patent #7,928,203). This work was supported by a collaborative research grant from Elan Pharmaceuticals, South San Francisco, USA; however, this does not alter the authors' adherence to PLOS ONE policies including data sharing and materials.
Figures
Figure 1. Binding of mAb 2A4 to synthetic AL-derived amyloid fibrils.
A) Concentration-dependent binding of mAb 2A4 assessed by SPR analyses. B&C) EuLISA of mAb 2A4 (closed circles) or control mAb JH70 (open circles) concentration dependent binding for rVλ6 Wil (B) or Jto (C) synthetic fibrils coated at 0.83 µM. (D) SPR analysis of mAb 2A4 binding to rVλ6Wil fibrils with addition of the immunizing peptide (2A4+immunogen), control reagent (2A4+peptide) or alone (2A4). (E) Binding of mAb2A4 to rVλ6Wil fibrils coated at 0.83 µM (-) or with addition of Wil fibrils in solution (Fib) or Wil monomer (Mon).
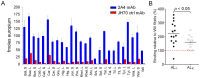
Figure 2. EuLISA of 2A4 (blue) or control JH70 (red) mAb at 50 nM binding to human AL amyloid extracts.
(A) Each amyloid extract derived from the liver (L) or spleen (S) was dried onto the well of a 96-well microplate normalized to the ThT signal equivalent to that of 10 µg/mL Hig, L. The far right bars of panel A show binding to synthetic WIL fibrils normalized to the same ThT value. (B) Summary of 2A4 binding to ALκ or ALλ amyloids, normalized to the binding to rVλ6WIL synthetic fibrils.
Figure 3. Immunohistochemistry of 2A4 binding to human ALκ and ALλ amyloid in tissue samples.
2A4 mAb was used to immunostain AL amyloid (arrows) in formalin-fixed paraffin-embedded tissues derived from the liver, kidney or pancreas. Top panels show staining with mAb 2A4 alone; middle panels show results with mAb 2A4 adsorbed with immunogen peptide -GHEDT- (imm) and bottom panels mAb 2A4 adsorbed with control peptide -GHETMADQE- (ctrl).
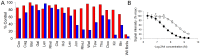
Figure 4. Binding of 2A4 to ALκ Hig amyloid extract and rVλ6Wil fibrils in the presence of different Bence Jones proteins.
(A) ALκ amyloid extract, 10 µg/mL Hig, L (blue) or 0.83 µM rVλ6Wil fibrils (red) were dried onto the wells of a 96-well microplate and the binding of 2A4 evaluated in the presence of BJp pre-incubated with 2A4. Concentrations of 2A4 were 5 nM for wells containing rVλ6 Wil (red) and 200 nM for the Hig samples (blue). Competitor BJps, designated on the abscissa by 3 letter code were present at 41.5 µM. The data are expressed as the % of the positive control, i.e., mAb 2A4 in the absence of any BJp. Competition with rVλ6 Wil fibrils (far right) served as a positive control. (B) EuLISA titration of 2A4 on Hig, L amyloid extract, 10 µg/mL (closed) and rVλ6 Wil fibrils, 0.25 µM (open). The EC50 for Wil was ∼3 nM and for Hig, ∼40 nM.
Figure 5. Evaluation of size-fractionated Kir BJp preparation as competitors.
(A) Absorbance profile of size fractionated soluble Kir BJp preparation using a Superdex 75 gel filtration column. (B) Fractions, 46, 52, 59 and 66, selected on the basis of the A280 chromatogram trace, were analyzed by SDS-PAGE, and then (C) tested for their ability to inhibit the reaction between 2A4 (200 nM) and Hig L amyloid extract (10 µg/mL) in the standard ELISA. Each fraction was used at the same concentration based on A280 to inhibit binding as described for Fig. 4A.
Figure 6. Binding of radioiodinated 2A4 or isotype matched control IgG MOPC 141 to human ALκ and ALλ amyloidomas.
Antibodies were radiolabeled with 125I and injected iv in the lateral tail vein (∼150 µCi, 25 µg) of mice bearing human AL amyloidomas (50 mg of human κ1 AL [Hig] or λ2 AL [Shi]), at 7 days post-implantation. Mice were imaged post mortem at 48 h post injection using SPECT/CT and the site of the amyloidoma was determined from the CT imaging and is indicated by the arrows (left panels). Tissues including the amyloid mass were harvested, fixed and sectioned for analysis. The right hand panels show autoradiography of the radioiodine tracer (ARG), hematoxylin and eosin staining for tissue morphology (H&E) or staining with Congo red to show amyloid deposits (CR).
Figure 7. Effect of mAb 2A4 on the resolution of human ALκ amyloidomas in mice.
The mAbs 2A4 or JH70 were administered subcutaneously into scid mice bearing human ALκ amyloidomas (50 mg Hig extract), 7 days after injection. Mice were euthanized and the mass of the residual amyloidoma was measured (A) and the amyloidomas photographed (B). Amyloidomas were fixed and processed for paraffin sectioning. Panel C shows immunohistochemical staining for macrophages (anti-Iba-1) in representative sections of amyloidomas from mice treated either with mAbs 2A4 or JH70. Arrowheads designate the positive staining for macrophages; arrows are the band of polymorphonuclear cells, and; “A” designates the major mass of amyloid material.
Similar articles
- 2A4 binds soluble and insoluble light chain aggregates from AL amyloidosis patients and promotes clearance of amyloid deposits by phagocytosis †.
Renz M, Torres R, Dolan PJ, Tam SJ, Tapia JR, Li L, Salmans JR, Barbour RM, Shughrue PJ, Nijjar T, Schenk D, Kinney GG, Zago W. Renz M, et al. Amyloid. 2016 Sep;23(3):168-177. doi: 10.1080/13506129.2016.1205974. Epub 2016 Aug 5. Amyloid. 2016. PMID: 27494229 Free PMC article. - Bifunctional amyloid-reactive peptide promotes binding of antibody 11-1F4 to diverse amyloid types and enhances therapeutic efficacy.
Wall JS, Williams AD, Foster JS, Richey T, Stuckey A, Macy S, Wooliver C, Campagna SR, Tague ED, Farmer AT, Lands RH, Martin EB, Heidel RE, Kennel SJ. Wall JS, et al. Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):E10839-E10848. doi: 10.1073/pnas.1805515115. Epub 2018 Oct 30. Proc Natl Acad Sci U S A. 2018. PMID: 30377267 Free PMC article. - Radioimaging of light chain amyloid with a fibril-reactive monoclonal antibody.
Wall JS, Kennel SJ, Paulus M, Gregor J, Richey T, Avenell J, Yap J, Townsend D, Weiss DT, Solomon A. Wall JS, et al. J Nucl Med. 2006 Dec;47(12):2016-24. J Nucl Med. 2006. PMID: 17138745 Free PMC article. - 124I/125I-Fibril-reactive monoclonal antibody.
Cheng KT. Cheng KT. 2006 Dec 7 [updated 2008 Jan 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2006 Dec 7 [updated 2008 Jan 2]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641290 Free Books & Documents. Review. - Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies.
Solomon A, Weiss DT, Wall JS. Solomon A, et al. Cancer Biother Radiopharm. 2003 Dec;18(6):853-60. doi: 10.1089/108497803322702824. Cancer Biother Radiopharm. 2003. PMID: 14969598 Review.
Cited by
- 2A4 binds soluble and insoluble light chain aggregates from AL amyloidosis patients and promotes clearance of amyloid deposits by phagocytosis †.
Renz M, Torres R, Dolan PJ, Tam SJ, Tapia JR, Li L, Salmans JR, Barbour RM, Shughrue PJ, Nijjar T, Schenk D, Kinney GG, Zago W. Renz M, et al. Amyloid. 2016 Sep;23(3):168-177. doi: 10.1080/13506129.2016.1205974. Epub 2016 Aug 5. Amyloid. 2016. PMID: 27494229 Free PMC article. - Status and Future Directions of Therapeutics and Prognosis of Cardiac Amyloidosis.
Zhang W, Ding J, Wang W, Wang D, Pan Y, Xu D. Zhang W, et al. Ther Clin Risk Manag. 2023 Jul 10;19:581-597. doi: 10.2147/TCRM.S414821. eCollection 2023. Ther Clin Risk Manag. 2023. PMID: 37457506 Free PMC article. Review. - Understanding Mesangial Pathobiology in AL-Amyloidosis and Monoclonal Ig Light Chain Deposition Disease.
Herrera GA, Teng J, Turbat-Herrera EA, Zeng C, Del Pozo-Yauner L. Herrera GA, et al. Kidney Int Rep. 2020 Jul 21;5(11):1870-1893. doi: 10.1016/j.ekir.2020.07.013. eCollection 2020 Nov. Kidney Int Rep. 2020. PMID: 33163710 Free PMC article. Review. - Doxycycline Plus Bortezomib-Containing Regimens for the Treatment of Light-Chain Amyloidosis in the Frontline Setting: Experience from the Amyloidosis Program of Calgary.
Lewis E, Fine N, McCulloch S, Tay J, Duggan P, Neri P, Bahlis N, Jimenez-Zepeda VH. Lewis E, et al. Curr Oncol. 2024 Sep 18;31(9):5608-5616. doi: 10.3390/curroncol31090415. Curr Oncol. 2024. PMID: 39330043 Free PMC article. - Bifunctional amyloid-reactive peptide promotes binding of antibody 11-1F4 to diverse amyloid types and enhances therapeutic efficacy.
Wall JS, Williams AD, Foster JS, Richey T, Stuckey A, Macy S, Wooliver C, Campagna SR, Tague ED, Farmer AT, Lands RH, Martin EB, Heidel RE, Kennel SJ. Wall JS, et al. Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):E10839-E10848. doi: 10.1073/pnas.1805515115. Epub 2018 Oct 30. Proc Natl Acad Sci U S A. 2018. PMID: 30377267 Free PMC article.
References
- Merlini G, Bellotti V (2003) Molecular mechanisms of amyloidosis. N Engl J Med 349: 583–596. - PubMed
- Dispenzieri A, Gertz MA, Buadi F (2012) What do I need to know about immunoglobulin light chain (AL) amyloidosis? Blood Rev 26: 137–154. - PubMed
- Mekinian A, Lions C, Leleu X, Duhamel A, Lamblin N, et al. (2010) Prognosis assessment of cardiac involvement in systemic AL amyloidosis by magnetic resonance imaging. Am J Med 123: 864–868. - PubMed
- Chee CE, Lacy MQ, Dogan A, Zeldenrust SR, Gertz MA (2010) Pitfalls in the diagnosis of primary amyloidosis. Clin Lymphoma Myeloma Leuk 10: 177–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by a collaborative research grant from Elan Pharmaceuticals, South San Francisco, USA (“funders”). The funders had no role in data collection and analysis associated with this manuscript. The decision to publish (and where) as well as certain aspects of the experimental design, were made jointly by JSW, SJK, PS, and DS.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases