Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth - PubMed (original) (raw)
Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth
Sabrina Malik et al. J Neurosci. 2013.
Abstract
Premature infants exhibit neurodevelopmental delay and reduced growth of the cerebral cortex. However, the underlying mechanisms have remained elusive. Therefore, we hypothesized that neurogenesis in the ventricular and subventricular zones of the cerebral cortex would continue in the third trimester of pregnancy and that preterm birth would suppress neurogenesis. To test our hypotheses, we evaluated autopsy materials from human fetuses and preterm infants of 16-35 gestational weeks (gw). We noted that both cycling and noncycling Sox2(+) radial glial cells and Tbr2(+) intermediate progenitors were abundant in human preterm infants until 28 gw. However, their densities consistently decreased from 16 through 28 gw. To determine the effect of premature birth on neurogenesis, we used a rabbit model and compared preterm [embryonic day 29 (E29), 3 d old] and term (E32, <2 h old) pups at an equivalent postconceptional age. Glutamatergic neurogenesis was suppressed in preterm rabbits, as indicated by the reduced number of Tbr2(+) intermediate progenitors and the increased number of Sox2(+) radial glia. Additionally, hypoxia-inducible factor-1α, vascular endothelial growth factor, and erythropoietin were higher in term than preterm pups, reflecting the hypoxic intrauterine environment of just-born term pups. Proneural genes, including Pax6 and Neurogenin-1 and -2, were higher in preterm rabbit pups compared with term pups. Importantly, neurogenesis and associated factors were restored in preterm pups by treatment with dimethyloxallyl glycine, a hypoxia mimetic agent. Hence, glutamatergic neurogenesis continues in the premature infants, preterm birth suppresses neurogenesis, and hypoxia-mimetic agents might restore neurogenesis, enhance cortical growth, and improve neurodevelopmental outcome of premature infants.
Figures
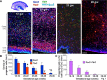
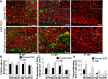
Figure 1.
Neurogenesis continues in dorsal SVZ until 28 gw. A, Representative immunofluorescence of cryosections from subjects of 17, 19, 23, and 26 gw triple labeled with Sox2, Tbr2, and Ki67 antibodies. There was an abundance of Sox2+ and Tbr2+ neuronal progenitors in the VZ and SVZ that decrease in density with increasing gestational age. A cresyl violet-stained coronal section from 17 gw forebrain (top left) shows dorsal cortical SVZ (green circle) and ganglionic eminence (asterisk); all images were acquired in dorsal SVZ. Scale bars, 100 μm. B, Densities of Sox2+ and Tbr2+ cells progressively reduce with advancing gestational age, becoming scarce after 28 gw. Bar charts are mean ± SEM. *p < 0.001, †p = 0.026, and ‡p = 0.047 for the comparison of Sox2+ cells between 29–35 gw versus 16–19, 20–22, and 26–28 gw, respectively. #p < 0.001 and δ_p_ < 0.001 each for the comparison of Tbr2+ cells between 16–19 versus 20–22 gw and 20–22 versus 23–25 gw. ψ_p_ < 0.03 for the comparison of Tbr2+ cells between 23–25 versus 26–28 gw. C, Bar charts are mean ± SEM. Neuronal progenitors coexpressing Sox2 and Tbr2 significantly decline in density with increasing gestational age, disappearing by 28 gw.
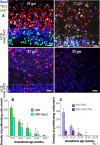
Figure 2.
Proliferation of apical and basal progenitors reduce with advancing gestation. A, Typical morphology of radial glia (Sox2+) and IPCs (Tbr2+) in the VZ and SVZ of cryosections taken from 19, 23, 27, and 33 gw subjects triple labeled with Sox2, Tbr2, and Ki67 antibodies. The number of cycling and noncycling Sox2+ and Tbr2+ cells reduces with increasing gestation, becoming completely absent by 33 gw. Scale bars, 25 μm. The dashed lines indicate the intersection of inner and outer SVZ. B, Densities of all proliferating cells and cycling Sox2+ cells decreased with advancing gestational age. Bar charts are mean ± SEM. *p < 0.001, †p < 0.001, and ‡p < 0.001 for the comparison of all proliferating cells (Ki67+) for 16–19 versus 20–22 gw, 16–19 versus 23–25 gw and 20–22 versus 26–28 gw, respectively. #p < 0.001 and δ_p_ < 0.001 for Sox2+ cells comparing 16–19 versus 20–22 gw and 16–19 versus 23–25 gw, respectively. ψ_p_ = 0.024 for Sox2+ cells comparing 20–22 versus 26–28 gw, respectively. C, Densities of cycling cells labeled Tbr2 and colabeled Sox2+ and Tbr2+ decreased with rising gestational age. Bar charts are mean ± SEM. *p < 0.001 and †p < 0.001 for the comparison of proliferating Tbr2+ cells for both 16–19 versus 20–22 gw and 16–19 versus 23–25 gw, p < 0.001. ‡p = 0.008 for the comparison of cycling Tbr2+ cells at 20–22 versus 26–28 gw.
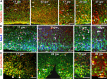
Figure 3.
Sox2+ radial glial cells show apical and basal processes in fetuses and premature infants. A, Double immunolabeling at 17, 23, and 27 gw with Sox2 and vimentin antibodies. Note the basal radial glial processes in outer SVZ (arrow) and predominance of Sox2+ cells (arrowhead) in VZ and inner SVZ. Sox2+ cells were embedded into vimentin. Scale bars, 25 μm. B, Triple immunolabeling of cryosections from 17 and 23 gw subjects with Sox2, nestin, and Ki67 antibodies. Nestin-positive radial glial processes surround the Sox2-positive (arrow) and Ki67-positive (arrowhead) nuclear signals. Scale bars, 25 μm. C, In 17 and 23 gw subjects, double labeling with Sox2 and p-vimentin antibodies shows colocalization of two immunoreactivities at several locations (arrowheads), indicating that the radial glial cells are in M phase. Note the processes of p-vimentin+ cells extending into SVZ (arrows). Scale bars, 25 μm.
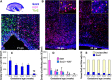
Figure 4.
Neurogenesis in ganglionic eminence continues until 28 gw. A, Representative immunofluorescence of cryosections from subjects of 21 gw, triple labeled with Sox2, Tbr2, and Ki67 antibodies. Note that Tbr2+ cells (arrowheads) are absent in the ganglionic eminence; the dashed line marks a sharp demarcation in the SVZ between dorsal (cortical) SVZ and ventrally located ganglionic eminence with respect to the distribution of Tbr2+ cells. Scale bar, 50 μm. Cresyl violet-stained coronal section from 17 gw (top left) forebrain shows ganglionic eminence (red circle), dorsal cortical SVZ (asterisk), and lateral ventricle (V). Images in B were acquired in the ganglionic eminence. B, Typical distribution of Sox2+ and Ki67+ cells in the ganglionic eminence of 19 and 23 gw infants. Note lesser density of Sox2+ cells in 23 gw relative to 19 gw. Arrowheads indicate cycling Sox2+ cells. Scale bar, 100 μm. C, Note decreased density of Sox2+ cells in lower age groups. Bar charts are mean ± SEM. *p < 0.001 for 16–19 versus 23–25 gw; †p = 0.017 for 20–22 versus 29–35 gw; ‡p = 0.013 for 26–28 versus 29–35 gw. D, All cycling neural cells and proliferating Sox2+ cells decreased in density with advancing gestational age. Bar charts are mean ± SEM. The following applies for all cycling cells: *p < 0.007 for 16–19 versus 20–22 gw; †p < 0.01 and ‡p < 0.01 for 16–19 versus 23–25 and 26–28 gw, respectively. The following applies for proliferating Sox2+ cells: #p < 0.001, δ_p_ < 0.001, and ψ_p_ < 0.001 for 16–19 vs 20–22, 26–28, and 29–35 gw, respectively. E, Proportional bar diagram showing reduction in the density of percentage of Sox+ cells with advancing gestational age.
Figure 5.
DCX+ cells are similar in density across gestational age categories in both the ganglionic eminence and dorsal SVZ. A, Representative immunofluorescence of cryosections from subjects of 19, 23, and 27 gw double labeled with DCX and Ki67 antibodies. Note that densities of DCX+ cells are comparable between the 19, 23, and 27 gw subjects in both dorsal SVZ (top) and ganglioic eminence (bottom), whereas the number of proliferating cells (Ki67+) is greatly reduced during the same period. Scale bars, 25 μm. B, Note that percentages of DCX+ cells (ratio of DCX+ and sytox+) are comparable across the gestational age categories in both dorsal SVZ and ganglionic eminence. Bar charts are mean ± SEM. C, The total number of DCX+ cells was comparable across gestational age categories in both the dorsal SVZ and ganglionic eminence. Bar charts are mean ± SEM. D, The number of proliferating DCX+ (DCX+ and Ki67+) cells significantly reduced as a function of gestational age in ganglionic eminence. However, in the dorsal SVZ, cycling DCX+ cells showed a trend toward reduction in density with advancing gestation, which was not significant. Bar charts are mean ± SEM. In ganglionic eminence for proliferating cells DCX+ cells, *p = 0.03 for 23–25 versus 25–28 gw and †p = 0.02 for 23–25 versus 29–35 gw.
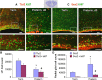
Figure 6.
Preterm birth in rabbits suppressed Tbr2+ IPCs and increased Sox2+ radial glia cells. A, Representative immunofluorescence of the dorsal SVZ from 3-d-old preterm pups and just-born term pups double labeled with Tbr2 and Ki67 antibodies. Bottom, Higher magnification. Note the higher density of both proliferating and nonproliferating Tbr2+ cells in term pups compared with preterm ones. Scale bars, 25 μm. A cresyl violet-stained coronal section from the forebrain of E29 rabbit pup (top right) shows dorsal cortical SVZ (boxes). B, The total number of Tbr2+ and cycling Tbr2+ cells were higher in term pups compared with preterm pups in dorsal VZ and SVZ. Bar charts are mean ± SEM (n = 5 each group). *p < 0.02 and #p = 0.01 for the comparison between preterm and term pups. C, Cryosections from 3-d-old preterm pups and immediately born term pups were double labeled with Sox2 and Ki67 antibodies. Low (top) and high (bottom) magnification of the dorsal SVZ are shown. Total and proliferating Sox2+ cells are less abundant in term pups compared with preterm ones. Scale bars, 25 μm. D, Data are mean ± SEM (n = 5 each group). The total and proliferating Sox2+ cells were fewer in term pups compared with preterm pups. *p < 0.05 and #p < 0.05 for the comparison between preterm and term pups.
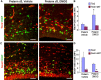
Figure 7.
DMOG treatment reversed glutamergic neurogenesis in preterm pups. A, C, Representative immunofluorescence of cryosections of dorsal VZ/SVZ from 3-d-old preterm pups treated either with DMOG or with vehicle. Sections were double labeled with Tbr2 and Ki67 (A) or Sox2 and Ki67 (C) antibodies. Note the higher density of Tbr2+ cells and lower density of Sox2+ in DMOG-treated pups compared with vehicle controls. Scale bars, 25 μm. B, Quantification of total and cycling Tbr2+ cells in DMOG- and vehicle-treated preterm pups. Bar charts are mean ± SEM (n = 5 each group). *p < 0.05 for the comparison between DMOG- and vehicle-treated preterm pups. D, The proliferating Sox2+ cells were fewer in DMOG-treated pups compared with vehicle controls. Data are mean ± SEM (n = 5 each group). *p < 0.05 for the comparison between DMOG- and vehicle-treated preterm pups.
Figure 8.
Reduced HIF-1α, EPO, and VEGF in preterm versus term pups, and DMOG elevates them. A, Note that EPO mRNA expression was reduced in preterm pups compared with term pups. VEGF mRNA accumulation showed a trend toward decrease in preterm pups relative to term pups. DMOG treatment elevated the levels of both EPO and VEGF. Data are mean ± SEM (n = 5 each group). B, Representative Western blot analyses for VEGF and HIF-1α for term, untreated preterm, and DMOG-treated preterm pups. VEGF and HIF1α protein levels were significantly reduced in preterm infants than term pups, and DMOG treatment increased the levels. Data are mean ± SEM (n = 5 each group). Values are normalized to β-actin levels. *p < 0.05 and **p < 0.01 for the comparison between preterm and term pups. #p < 0.05 and ##p < 0.01 for the comparison between untreated and DMOG-treated preterm pups.
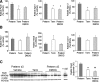
Figure 9.
Preterm birth elevates levels of Pax6 and neurogenin genes, and DMOG treatment restores them. A, Data are mean ± SEM (n = 5 each group). Note that Pax6 and Ngn 2 expression was significantly elevated in preterm pups compared with term pups. DMOG treatment in preterm pups showed a trend toward decrease relative to untreated preterm pups for both Pax6 and Ngn2. Ngn1 gene expression was also significantly higher in preterm pups relative to term pups, and DMOG treatment reduced the level. B, Hes1/5, Emx1/2, and Insm1 were similar between the three groups as indicated. C, Representative Western blot analyses for Pax6 for term, untreated preterm, and DMOG-treated preterm pups. Data are mean ± SEM (n = 5 each group). Values are normalized to β-actin levels. Pax6 levels were higher in preterm pups compared with term pups, and DMOG treatment significantly reduced Pax6 levels. *p < 0.05 and **p < 0.01 for the comparison between preterm and term pups. #p < 0.05 and ##p < 0.01 for the comparison between untreated and DMOG treated preter pups.
Similar articles
- Disruption of Interneuron Neurogenesis in Premature Newborns and Reversal with Estrogen Treatment.
Tibrewal M, Cheng B, Dohare P, Hu F, Mehdizadeh R, Wang P, Zheng D, Ungvari Z, Ballabh P. Tibrewal M, et al. J Neurosci. 2018 Jan 31;38(5):1100-1113. doi: 10.1523/JNEUROSCI.1875-17.2017. Epub 2017 Dec 15. J Neurosci. 2018. PMID: 29246927 Free PMC article. - Prenatal betamethasone does not affect glutamatergic or GABAergic neurogenesis in preterm newborns.
Vose LR, Vinukonda G, Diamond D, Korumilli R, Hu F, Zia MT, Hevner R, Ballabh P. Vose LR, et al. Neuroscience. 2014 Jun 13;270:148-57. doi: 10.1016/j.neuroscience.2014.04.009. Epub 2014 Apr 13. Neuroscience. 2014. PMID: 24735821 Free PMC article. - Estrogen Treatment Reverses Prematurity-Induced Disruption in Cortical Interneuron Population.
Panda S, Dohare P, Jain S, Parikh N, Singla P, Mehdizadeh R, Klebe DW, Kleinman GM, Cheng B, Ballabh P. Panda S, et al. J Neurosci. 2018 Aug 22;38(34):7378-7391. doi: 10.1523/JNEUROSCI.0478-18.2018. Epub 2018 Jul 23. J Neurosci. 2018. PMID: 30037831 Free PMC article. - [Late preterm : high risk newborns despite appearances].
Snyers D, Lefebvre C, Viellevoye R, Rigo V. Snyers D, et al. Rev Med Liege. 2020 Feb;75(2):105-110. Rev Med Liege. 2020. PMID: 32030935 Review. French. - Ontogeny of autonomic regulation in late preterm infants born at 34-37 weeks postmenstrual age.
Hunt CE. Hunt CE. Semin Perinatol. 2006 Apr;30(2):73-6. doi: 10.1053/j.semperi.2006.02.005. Semin Perinatol. 2006. PMID: 16731280 Review.
Cited by
- Assessment of radial glia in the frontal lobe of fetuses with Down syndrome.
Baburamani AA, Vontell RT, Uus A, Pietsch M, Patkee PA, Wyatt-Ashmead J, Chin-Smith EC, Supramaniam VG, Donald Tournier J, Deprez M, Rutherford MA. Baburamani AA, et al. Acta Neuropathol Commun. 2020 Aug 20;8(1):141. doi: 10.1186/s40478-020-01015-3. Acta Neuropathol Commun. 2020. PMID: 32819430 Free PMC article. - Microstructural Periventricular White Matter Injury in Post-hemorrhagic Ventricular Dilatation.
Isaacs AM, Neil JJ, McAllister JP, Dahiya S, Castaneyra-Ruiz L, Merisaari H, Botteron HE, Alexopoulos D, George A, Sun P, Morales DM, Shimony JS, Strahle J, Yan Y, Song SK, Limbrick DD, Smyser CD. Isaacs AM, et al. Neurology. 2022 Jan 24;98(4):e364-e375. doi: 10.1212/WNL.0000000000013080. Neurology. 2022. PMID: 34799460 Free PMC article. - Post-transcriptional regulatory elements and spatiotemporal specification of neocortical stem cells and projection neurons.
DeBoer EM, Kraushar ML, Hart RP, Rasin MR. DeBoer EM, et al. Neuroscience. 2013 Sep 17;248:499-528. doi: 10.1016/j.neuroscience.2013.05.042. Epub 2013 May 30. Neuroscience. 2013. PMID: 23727006 Free PMC article. Review. - Greater Number of Microglia in Telencephalic Proliferative Zones of Human and Nonhuman Primate Compared with Other Vertebrate Species.
Penna E, Cunningham CL, Saylor S, Kreutz A, Tarantal AF, Martínez-Cerdeño V, Noctor SC. Penna E, et al. Cereb Cortex Commun. 2021 Sep 6;2(4):tgab053. doi: 10.1093/texcom/tgab053. eCollection 2021. Cereb Cortex Commun. 2021. PMID: 34647030 Free PMC article. - Cerebral Lateralization is Protective in the Very Prematurely Born.
Scheinost D, Lacadie C, Vohr BR, Schneider KC, Papademetris X, Constable RT, Ment LR. Scheinost D, et al. Cereb Cortex. 2015 Jul;25(7):1858-66. doi: 10.1093/cercor/bht430. Epub 2014 Jan 22. Cereb Cortex. 2015. PMID: 24451659 Free PMC article. Clinical Trial.
References
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. - PubMed
- Bel-Vialar S, Medevielle F, Pituello F. The on/off of Pax6 controls the tempo of neuronal differentiation in the developing spinal cord. Dev Biol. 2007;305:659–673. - PubMed
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources