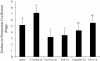Dietary oil composition differentially modulates intestinal endotoxin transport and postprandial endotoxemia - PubMed (original) (raw)
Dietary oil composition differentially modulates intestinal endotoxin transport and postprandial endotoxemia
Venkatesh Mani et al. Nutr Metab (Lond). 2013.
Abstract
Background: Intestinal derived endotoxin and the subsequent endotoxemia can be considered major predisposing factors for diseases such as atherosclerosis, sepsis, obesity and diabetes. Dietary fat has been shown to increase postprandial endotoxemia. Therefore, the aim of this study was to assess the effects of different dietary oils on intestinal endotoxin transport and postprandial endotoxemia using swine as a model. We hypothesized that oils rich in saturated fatty acids (SFA) would augment, while oils rich in n-3 polyunsaturated fatty acids (PUFA) would attenuate intestinal endotoxin transport and circulating concentrations.
Methods: Postprandial endotoxemia was measured in twenty four pigs following a porridge meal made with either water (Control), fish oil (FO), vegetable oil (VO) or coconut oil (CO). Blood was collected at 0, 1, 2, 3 and 5 hours postprandial and measured for endotoxin. Furthermore, ex vivo ileum endotoxin transport was assessed using modified Ussing chambers and intestines were treated with either no oil or 12.5% (v/v) VO, FO, cod liver oil (CLO), CO or olive oil (OO). Ex vivo mucosal to serosal endotoxin transport permeability (Papp) was then measured by the addition of fluorescent labeled-lipopolysaccharide.
Results: Postprandial serum endotoxin concentrations were increased after a meal rich in saturated fatty acids and decreased with higher n-3 PUFA intake. Compared to the no oil control, fish oil and CLO which are rich in n-3 fatty acids reduced ex vivo endotoxin Papp by 50% (P < 0.05). Contrarily, saturated fatty acids increased the Papp by 60% (P = 0.008). Olive and vegetable oils did not alter intestinal endotoxin Papp.
Conclusion: Overall, these results indicate that saturated and n-3 PUFA differentially regulate intestinal epithelial endotoxin transport. This may be associated with fatty acid regulation of intestinal membrane lipid raft mediated permeability.
Figures
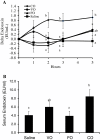
Figure 1
Dietary oil alters postprandial serum endotoxin concentrations in pigs fed a single dietary oil-based meal. A) Delta change in serum endotoxin concentrations. B) Mean postprandial serum endotoxin concentration. Different letters (a,b) represent significant difference at P < 0.05. Treatments are a porridge meal made with either no oil (saline), fish oil (FO), vegetable oil (VO) and coconut oil (CO). n = 6 pigs/treatment. Data are means ± S.E.M.
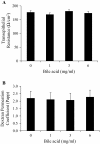
Figure 2
The effect of increasing porcine bile acid concentration on ex vivo intestinal integrity and permeability. A) Transepithelial resistance (TER) and B) FITC-Dextran transport (4.4 kDa). Freshly isolated ileum samples were mounted into modified Ussing chambers and incubated with the indicated concentration of bile acid for 30 minutes and then FITC-Dextran was added to mucosal side. Permeation coefficient was calculated by taking samples from chambers every 10–15 minutes and measuring the amount of fluorescence. Different letters represent significant difference at P < 0.05. n = 11 pigs. Data are means ± S.E.M.
Figure 3
Ex vivo endotoxin transport in pig ileum tissue exposed to different dietary oil treatments. Freshly isolated ileum samples were mounted into modified Ussing chambers and mixed with the indicated oils and 20 mM bile acid for 120 minutes and FITC-LPS transport was measured. Different letters represent significant difference at P < 0.05. n = 11 per treatment. Data are means ± S.E.M.
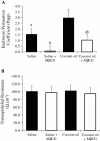
Figure 4
Lipid raft modifier methyl beta cyclodextrin (MβCD) decreases ex vivo endotoxin transport. A) Endotoxin transport and B) transepithelial resistance was measured using Ussing chambers in ileum tissues treated with either control (water), MβCD, coconut oil, or coconut oil plus MβCD. Tissue (n = 7 /trt) were pretreated with these treatments for 30 min before FITC-LPS transport was assessed. Different letters represent significant difference at P < 0.05. Data are means ± S.E.M.
Similar articles
- Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross-over study.
Lyte JM, Gabler NK, Hollis JH. Lyte JM, et al. Lipids Health Dis. 2016 Nov 5;15(1):186. doi: 10.1186/s12944-016-0357-6. Lipids Health Dis. 2016. PMID: 27816052 Free PMC article. Clinical Trial. - Postprandial lipaemia following consumption of a meal enriched with medium chain saturated and/or long chain omega-3 polyunsaturated fatty acids. A randomised cross-over study.
Austin G, Ferguson JJ, Thota RN, Singh H, Burrows T, Garg ML. Austin G, et al. Clin Nutr. 2021 Feb;40(2):420-427. doi: 10.1016/j.clnu.2020.06.027. Epub 2020 Jul 1. Clin Nutr. 2021. PMID: 32684486 Clinical Trial. - An acute intake of a walnut-enriched meal improves postprandial adiponectin response in healthy young adults.
Lozano A, Perez-Martinez P, Marin C, Tinahones FJ, Delgado-Lista J, Cruz-Teno C, Gomez-Luna P, Rodriguez-Cantalejo F, Perez-Jimenez F, Lopez-Miranda J. Lozano A, et al. Nutr Res. 2013 Dec;33(12):1012-8. doi: 10.1016/j.nutres.2013.08.010. Epub 2013 Oct 9. Nutr Res. 2013. PMID: 24267040 Clinical Trial. - The effects of dietary oils on the fatty acid composition and osmotic fragility of rat erythrocytes.
Kirchgessner M, Stangl GI, Reichlmayr-Lais AM, Eder K. Kirchgessner M, et al. Z Ernahrungswiss. 1994 Jun;33(2):146-58. doi: 10.1007/BF01622227. Z Ernahrungswiss. 1994. PMID: 8079509 - Fat modification in the diabetes diet.
Julius U. Julius U. Exp Clin Endocrinol Diabetes. 2003 Apr;111(2):60-5. doi: 10.1055/s-2003-39230. Exp Clin Endocrinol Diabetes. 2003. PMID: 12746754 Review.
Cited by
- Targeting the NLRP3 inflammasome-IL-1β pathway in type 2 diabetes and obesity.
Meier DT, de Paula Souza J, Donath MY. Meier DT, et al. Diabetologia. 2024 Nov 4. doi: 10.1007/s00125-024-06306-1. Online ahead of print. Diabetologia. 2024. PMID: 39496966 Review. - Multiple sclerosis: a narrative overview of current pharmacotherapies and emerging treatment prospects.
Olejnik P, Roszkowska Z, Adamus S, Kasarełło K. Olejnik P, et al. Pharmacol Rep. 2024 Oct;76(5):926-943. doi: 10.1007/s43440-024-00642-0. Epub 2024 Aug 23. Pharmacol Rep. 2024. PMID: 39177889 Free PMC article. Review. - Gut microbes, diet, and genetics as drivers of metabolic liver disease: a narrative review outlining implications for precision medicine.
Hermanson JB, Tolba SA, Chrisler EA, Leone VA. Hermanson JB, et al. J Nutr Biochem. 2024 Nov;133:109704. doi: 10.1016/j.jnutbio.2024.109704. Epub 2024 Jul 17. J Nutr Biochem. 2024. PMID: 39029595 Review. - Effects of fat sources on liver characteristics and intestinal morphometry in an early-life animal model.
Gutiérrez Zorrilla IM, Bernuy-Osorio ND, Zea Mendoza O, Yabar Villanueva EF, Vílchez-Perales C. Gutiérrez Zorrilla IM, et al. Rev Peru Med Exp Salud Publica. 2023 Out-Dec;40(4):459-465. doi: 10.17843/rpmesp.2023.404.12804. Rev Peru Med Exp Salud Publica. 2023. PMID: 38597474 Free PMC article. - Ketogenic Diet High in Saturated Fat Promotes Colonic Claudin Expression without Changes in Intestinal Permeability to Iohexol in Healthy Mice.
Luiskari L, Launonen H, Lindén J, Lehto M, Vapaatalo H, Salmenkari H, Korpela R. Luiskari L, et al. Nutrients. 2023 Dec 20;16(1):18. doi: 10.3390/nu16010018. Nutrients. 2023. PMID: 38201850 Free PMC article.
References
- Laugerette F, Vors C, Geloen A, Chauvin MA, Soulage C, Lambert-Porcheron S, Peretti N, Alligier M, Burcelin R, Laville M. et al.Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem. 2011;22:53–59. doi: 10.1016/j.jnutbio.2009.11.011. - DOI - PubMed
- Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. - PubMed
- Ghanim H, Abuaysheh S, Sia CL, Korzeniewski K, Chaudhuri A, Fernandez-Real JM, Dandona P. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, High-Carbohydrate Meal. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. - DOI - PMC - PubMed
- Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources