Anti-inflammatory therapy in chronic disease: challenges and opportunities - PubMed (original) (raw)
Review
Anti-inflammatory therapy in chronic disease: challenges and opportunities
Ira Tabas et al. Science. 2013.
Abstract
A number of widespread and devastating chronic diseases, including atherosclerosis, type 2 diabetes, and Alzheimer's disease, have a pathophysiologically important inflammatory component. In these diseases, the precise identity of the inflammatory stimulus is often unknown and, if known, is difficult to remove. Thus, there is interest in therapeutically targeting the inflammatory response. Although there has been success with anti-inflammatory therapy in chronic diseases triggered by primary inflammation dysregulation or autoimmunity, there are considerable limitations. In particular, the inflammatory response is critical for survival. As a result, redundancy, compensatory pathways, and necessity narrow the risk:benefit ratio of anti-inflammatory drugs. However, new advances in understanding inflammatory signaling and its links to resolution pathways, together with new drug development, offer promise in this area of translational biomedical research.
Figures
Fig. 1
Inflammation in infection, injury, and chronic disease. (A) Acute tonsillitis. White patches represent the accumulation of neutrophils in the tonsils in response to bacterial infection. The tonsils are swollen, erythematous, and painful. Normal tissue homeostasis is usually restored over the course of several days upon eradication of the infection. [Source: Michaelbladon/Wikimedia Commons] (B) Sprained ankle. An example of a sterile tissue injury. Swelling and redness result from the inflammatory response to tissue damage and hemorrhage. Normal tissue homeostasis is usually restored over a time course of weeks after clearance of dead or damaged cells and activation of tissue repair programs. [Source: Boldie/ Wikimedia Commons] (C) Rheumatoid arthritis. An example of chronic inflammation in a disease triggered primarily by autoimmunity. Persistent inflammation, driven in part by TNFα, results in severe tissue damage, joint destruction, and loss of function. [Source: James Heilman, MD/Wikimedia Commons] (D) Cross section of a coronary artery at the location of an atherosclerotic lesion. An example of a chronic inflammatory disease that is triggered initially by a process other than infection, tissue injury, or autoimmunity. Elevated levels of apolipoprotein B–containing lipoproteins in the artery wall induce a chronic inflammatory state characterized by activated endothelial cells and recruitment and activation of macrophages and other immune cells. As lesions evolve, inflammation fails to resolve in the setting of persistent arterial-wall lipoproteins. Nonresolving inflammation leads to cell death and necrotic core formation; cycles of extracellular matrix deposition and degradation; and calcification. [Source: Nephron/Wikimedia Commons]
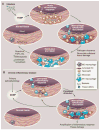
Fig. 2
Evolution of resolving versus nonresolving inflammation at a cellular level. (A) Typical features of a normal acute inflammatory response to infection that is detected by presentation of PAMPs to pattern recognition receptors. Eradication of the pathogen eliminates the stimulus, along with causing some reversible collateral tissue damage, and sets the stage for the resolution/repair phase, leading to restoration of normal tissue homeostasis. (B) Typical features of a chronic inflammatory disease caused by a nonimmune pathophysiologic process that in one way or another triggers an initial sterile inflammatory response, often indolent and likely through production of damage-associated molecular patterns (DAMPs). This initial response then becomes amplified by cytokines and chemokines. Because this response does not eradicate the initial stimulus, persistent nonresolving inflammation occurs, ultimately resulting in tissue damage. The inflammatory response itself may positively influence the production of DAMPS, which provides an additional positive feedback loop. For example, in the case of atherosclerosis, reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) may modify subendothelial lipoproteins in a manner that amplifies their ability to promote inflammation.
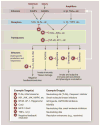
Fig. 3
Inflammation at a signaling level and candidate therapeutic targets. Inflammation is typically initiated by pattern recognition receptors, such as TLRs and NLRs, that recognize PAMPs and/or DAMPs. These receptors typically couple to signal transduction pathways that activate latent transcription factors that include members of the NF-κB and AP-1 families. These factors in turn act in a combinatorial and cell-specific manner to induce the expression of a large number of genes that exert antimicrobial activities—e.g., generate ROI and RNI. Chemokines regulate the recruitment of additional immune cells. Production of bioactive lipids, such as prostaglandins, also regulates pro- and anti-inflammatory cell functions. Expression of inflammatory cytokines provides a feed-forward loop for amplification of the initial response. The production of anti-inflammatory/ resolution mediators, such as IL-10 and PGE2, in response to proinflammatory signals suggests that resolution programs are an inherent aspect of inflammation. Letters in red “stop signs” represent examples of points in proinflammatory signaling pathways that are existing or potential targets for therapeutic intervention. Ab, antibody; GR, glucocorticoid receptor; HDM, histone demethylase; HMT, histone methyltransferase; IKK, IκB kinase; MAPK, mitogen-activated protein kinase; MCP1, monocyte chemotactic protein–1; TNFR, tumor necrosis factor receptor Cox2, cyclooxygenase 2; NSAIDs, nonsteroidal anti-inflammatory drugs.
Similar articles
- The Role of Chronic Inflammation in Various Diseases and Anti-inflammatory Therapies Containing Natural Products.
Wang RX, Zhou M, Ma HL, Qiao YB, Li QS. Wang RX, et al. ChemMedChem. 2021 May 18;16(10):1576-1592. doi: 10.1002/cmdc.202000996. Epub 2021 Mar 7. ChemMedChem. 2021. PMID: 33528076 Review. - Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease.
Esser N, Paquot N, Scheen AJ. Esser N, et al. Expert Opin Investig Drugs. 2015 Mar;24(3):283-307. doi: 10.1517/13543784.2015.974804. Epub 2014 Oct 25. Expert Opin Investig Drugs. 2015. PMID: 25345753 Review. - Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases.
Aggarwal BB, Harikumar KB. Aggarwal BB, et al. Int J Biochem Cell Biol. 2009 Jan;41(1):40-59. doi: 10.1016/j.biocel.2008.06.010. Epub 2008 Jul 9. Int J Biochem Cell Biol. 2009. PMID: 18662800 Free PMC article. Review. - Novel Applications of NSAIDs: Insight and Future Perspectives in Cardiovascular, Neurodegenerative, Diabetes and Cancer Disease Therapy.
Kaduševičius E. Kaduševičius E. Int J Mol Sci. 2021 Jun 21;22(12):6637. doi: 10.3390/ijms22126637. Int J Mol Sci. 2021. PMID: 34205719 Free PMC article. Review. - Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications.
Pollack RM, Donath MY, LeRoith D, Leibowitz G. Pollack RM, et al. Diabetes Care. 2016 Aug;39 Suppl 2:S244-52. doi: 10.2337/dcS15-3015. Diabetes Care. 2016. PMID: 27440839 Review.
Cited by
- Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles.
Kamaly N, Fredman G, Subramanian M, Gadde S, Pesic A, Cheung L, Fayad ZA, Langer R, Tabas I, Farokhzad OC. Kamaly N, et al. Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6506-11. doi: 10.1073/pnas.1303377110. Epub 2013 Mar 26. Proc Natl Acad Sci U S A. 2013. PMID: 23533277 Free PMC article. - Novel Endogenous Proresolving Molecules:Essential Fatty Acid-Derived and Gaseous Mediators in the Resolution of Inflammation.
Shinohara M, Serhan CN. Shinohara M, et al. J Atheroscler Thromb. 2016 Jun 1;23(6):655-64. doi: 10.5551/jat.33928. Epub 2016 Apr 6. J Atheroscler Thromb. 2016. PMID: 27052783 Free PMC article. Review. - Gentiana scabra Reduces SR-A Expression and Oxidized-LDL Uptake in Human Macrophages.
Lin CS, Liu PY, Lian CH, Lin CH, Lai JH, Ho LJ, Yang SP, Cheng SM. Lin CS, et al. Acta Cardiol Sin. 2016 Jul;32(4):460-6. doi: 10.6515/acs20150416a. Acta Cardiol Sin. 2016. PMID: 27471359 Free PMC article. - The Structural Basis for Nonsteroidal Anti-Inflammatory Drug Inhibition of Human Dihydrofolate Reductase.
Duff MR Jr, Gabel SA, Pedersen LC, DeRose EF, Krahn JM, Howell EE, London RE. Duff MR Jr, et al. J Med Chem. 2020 Aug 13;63(15):8314-8324. doi: 10.1021/acs.jmedchem.0c00546. Epub 2020 Jul 28. J Med Chem. 2020. PMID: 32658475 Free PMC article.
References
- Nathan C, Ding A. Cell. 2010;140:871. - PubMed
- Castellheim A, Brekke OL, Espevik T, Harboe M, Mollnes TE. Scand J Immunol. 2009;69:479. - PubMed
- Kubota T, Koike R. Mod Rheumatol. 2010;20:213. - PubMed
- McInnes IB, Schett G. N Engl J Med. 2011;365:2205. - PubMed
- Scrivo R, Vasile M, Bartosiewicz I, Valesini G. Autoimmun Rev. 2011;10:369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL054591/HL/NHLBI NIH HHS/United States
- HC088093/HC/NHLBI NIH HHS/United States
- R01 HL057560/HL/NHLBI NIH HHS/United States
- R01 DK091183/DK/NIDDK NIH HHS/United States
- R01 HL075662/HL/NHLBI NIH HHS/United States
- R01-HL075662/HL/NHLBI NIH HHS/United States
- DK091183/DK/NIDDK NIH HHS/United States
- P01 HL087123/HL/NHLBI NIH HHS/United States
- DK074868/DK/NIDDK NIH HHS/United States
- R01 HL106019/HL/NHLBI NIH HHS/United States
- R01 HL107497/HL/NHLBI NIH HHS/United States
- P01 DK074868/DK/NIDDK NIH HHS/United States
- R01-HL057560/HL/NHLBI NIH HHS/United States
- P01-HL054591/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials