p16INK4A represses breast stromal fibroblasts migration/invasion and their VEGF-A-dependent promotion of angiogenesis through Akt inhibition - PubMed (original) (raw)
p16INK4A represses breast stromal fibroblasts migration/invasion and their VEGF-A-dependent promotion of angiogenesis through Akt inhibition
Mysoon M Al-Ansari et al. Neoplasia. 2012 Dec.
Abstract
Stromal fibroblasts, the most abundant and probably the most active cellular component of breast cancer-associated stroma, become active and promote angiogenesis through paracrine effects. However, it still unclear how these processes are regulated. Here, we have shown that down-regulation of the tumor suppressor p16(INK4A) protein enhances the migration/invasion abilities of breast stromal fibroblasts, which form dendritic network of extensions into matrigel. Furthermore, we present clear evidence that p16(INK4A) represses the expression/secretion of the proangiogenesis protein vascular endothelial growth factor A (VEGF-A). Consequently, p16(INK4A)-deficient breast stromal fibroblasts and mouse embryonic fibroblasts enhanced endothelial cell differentiation into capillary-like structures in a paracrine manner. This effect was suppressed by adding bevacizumab, a specific VEGF-A inhibitor. Additionally, p16(INK4A)-defective mouse embryonic fibroblasts enhanced angiogenesis in breast cancer xenografts in mice. Furthermore, we have shown that p16(INK4A) suppresses the Akt/mammalian target of rapamycin (mTOR) signaling pathway and its downstream effector hypoxia-inducible factor 1-alpha (HIF-1α), which transactivates VEGF-A. Consequently, Akt inactivation suppressed both the p16(INK4A)-dependent autocrine effect on fibroblast migration/invasion and the paracrine effect on angiogenesis, showing the important role of this protein kinase in mediating the various effects related to p16(INK4A) deficiency. These results indicate that p16(INK4A) is an efficient inhibitor of the migration/invasion abilities of breast stromal fibroblasts and also their paracrine proangiogenic effects, through inhibition of Akt. Therefore, pharmacologic restoration of p16(INK4A) level in stromal fibroblasts may be exploited as therapeutic strategy to help eradicate tumor cells and/or prevent their recurrence, through suppressing cell non-autonomous procarcinogenic mediators.
Figures
Figure 1
p16 down-regulation enhances fibroblast migration and invasion. (A) Whole-cell lysates were prepared from TCF-64, CAF-64, T64si, and T64C cells and were used for immunoblot analysis. The numbers below the bands indicate fold of induction after normalization against GAPDH. (B, C) T64si and T64C cells (4 x 105) were cultured on the upper compartments of BioCoat Matrigel chambers in the presence of SFM. After 24 hours of incubation, cells were stained with Diff-Quick stain and then counted. (B) Representative photographs showing invasive and migrated cells. Scale bars represent 20 _µ_m. (C) Histograms depict average numbers of invasive and migrated cells, and error bars represent means ± SD. (D) Cells (6 x 104) were seeded with CpM in six-well plates coated with BD Matrigel basement membrane matrix for 24 hours. Representative photographs show the morphology of the invading cells. Scale bars represent 30 _µ_m.
Figure 2
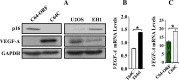
p16 suppresses the expression of VEGF-A. (A) Cell lysates were prepared from the indicated cells and used for immunoblot analysis using the indicated antibodies. (B, C) Total RNA was extracted from the indicated cells and the amount of the VEGF-A mRNA was assessed by real-time RT-PCR. Error bars represent means ± SD. *P < .001.
Figure 3
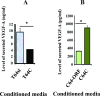
p16 represses VEGF-A secretion from breast stromal fibroblasts. (A, B) Conditioned media from the indicated cells were collected after 24 hours and the level of secreted VEGF-A was determined by ELISA. Error bars represent means ± SD. *P < .001.
Figure 4
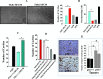
SFCM from p16-deficient human and mice fibroblasts activate HUVEC tube formation in a VEGF-A-dependent manner. SFCM were collected after 24 hours of incubation from the indicated cells and were added independently on HUVECs plated on matrigel (96-well plate), and the differentiation into capillary-like structures was assessed after 4 hours of incubation. (A) Representative photographs of HUVEC cavities. Scale bars represent 30 _µ_m. Stars show cavities and the circle indicates non-sprouting cells. (B) Histograms show number of cavities and non-sprouting cells. (C) SFCM were collected from MEF cells. (D) SFCM from T64si cells were treated with IgG1 or bevacizumab. The numbers between brackets indicate bevacizumab concentrations in _µ_M. (E) Breast cancer xenografts were created by co-injecting MDA-MB-231 cells with p16-/- or p16+/+ MEFs subcutaneously into nude mice. Immunohistochemistry was carried out on tumors containing MEFs either p16-/- or p16+/+ using anti-CD-31 antibody. Scale bars represent 30 _µ_m. Histogram shows average number of microvessels observed in five different fields from two different tumors. Error bars represent means ± SD. *P < .001.
Figure 5
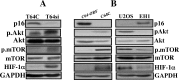
p16 represses the Akt/mTOR/HIF-1α pathway. (A, B) Whole cell lysates were prepared from the indicated cells and were used for immunoblot analysis using the indicated antibodies.
Figure 6
p16 represses stromal fibroblasts migration/invasion and their proangiogenic effect through Akt inhibition. (A) T64si cells were treated with Akt inhibitor III (10 µ_M) and DMSO (-) for 18 hours (upper panel) or cells were treated with control-siRNA and Akt-siRNA (lower panel), and whole-cell lysates were prepared and used for immunoblot analysis using the indicated antibodies. (B, D, E) T64si cells were treated either with Akt inhibitor III (10 µ_M) or DMSO. (B, D) The migration and invasion abilities were assessed using matrigel chambers as in Figure 1_C. Error bars represent means ± SD. *P < .001 (B), or BD matrigel basement membrane matrix as in Figure 1_D (D). (C) T64si cells were treated either with control-siRNA or Akt-siRNA, and the migration/invasion abilities of these cells were assessed as in Figure 1_C_. Representative photographs of migrating and invading cells are shown. Error bars represent means ± SD. (E) SFCM were collected and were added independently on HUVECs plated on matrigel (96-well plate), and the differentiation into capillary-like structures was assessed after 4 hours of incubation. Representative photographs of HUVEC cavities are shown. Scale bars represent 30 _µ_m.
Similar articles
- p16(INK4A) represses the paracrine tumor-promoting effects of breast stromal fibroblasts.
Al-Ansari MM, Hendrayani SF, Shehata AI, Aboussekhra A. Al-Ansari MM, et al. Oncogene. 2013 May 2;32(18):2356-64. doi: 10.1038/onc.2012.270. Epub 2012 Jul 2. Oncogene. 2013. PMID: 22751126 Free PMC article. - miR-146b-5p mediates p16-dependent repression of IL-6 and suppresses paracrine procarcinogenic effects of breast stromal fibroblasts.
Al-Ansari MM, Aboussekhra A. Al-Ansari MM, et al. Oncotarget. 2015 Oct 6;6(30):30006-16. doi: 10.18632/oncotarget.4933. Oncotarget. 2015. PMID: 26338965 Free PMC article. - Curcumin triggers p16-dependent senescence in active breast cancer-associated fibroblasts and suppresses their paracrine procarcinogenic effects.
Hendrayani SF, Al-Khalaf HH, Aboussekhra A. Hendrayani SF, et al. Neoplasia. 2013 Jun;15(6):631-40. doi: 10.1593/neo.13478. Neoplasia. 2013. PMID: 23730211 Free PMC article. - Autocrine functions of VEGF in breast tumor cells: adhesion, survival, migration and invasion.
Perrot-Applanat M, Di Benedetto M. Perrot-Applanat M, et al. Cell Adh Migr. 2012 Nov-Dec;6(6):547-53. doi: 10.4161/cam.23332. Epub 2012 Nov 1. Cell Adh Migr. 2012. PMID: 23257828 Free PMC article. Review. - Angiogenesis induction in breast cancer: A paracrine paradigm.
Badodekar N, Sharma A, Patil V, Telang G, Sharma R, Patil S, Vyas N, Somasundaram I. Badodekar N, et al. Cell Biochem Funct. 2021 Oct;39(7):860-873. doi: 10.1002/cbf.3663. Epub 2021 Sep 9. Cell Biochem Funct. 2021. PMID: 34505714 Review.
Cited by
- Overcoming intratumor heterogeneity of polygenic cancer drug resistance with improved biomarker integration.
Rehemtulla A. Rehemtulla A. Neoplasia. 2012 Dec;14(12):1278-89. doi: 10.1593/neo.122096. Neoplasia. 2012. PMID: 23308059 Free PMC article. - The protective effect of p16(INK4a) in oral cavity carcinomas: p16(Ink4A) dampens tumor invasion-integrated analysis of expression and kinomics pathways.
Isayeva T, Xu J, Ragin C, Dai Q, Cooper T, Carroll W, Dayan D, Vered M, Wenig B, Rosenthal E, Grizzle W, Anderson J, Willey CD, Yang ES, Brandwein-Gensler M. Isayeva T, et al. Mod Pathol. 2015 May;28(5):631-53. doi: 10.1038/modpathol.2014.149. Epub 2014 Dec 19. Mod Pathol. 2015. PMID: 25523612 - Crosstalk of methylation and tamoxifen in breast cancer (Review).
Shen J, He Y, Li S, Chen H. Shen J, et al. Mol Med Rep. 2024 Oct;30(4):180. doi: 10.3892/mmr.2024.13304. Epub 2024 Aug 12. Mol Med Rep. 2024. PMID: 39129315 Free PMC article. Review. - Expression of the tumor suppressor gene p16, and lymph node metastasis in patients with ovarian cancer.
Wang H, Zheng J, Li Q, Zhou M, Ai D, Zhang H. Wang H, et al. Oncol Lett. 2017 Oct;14(4):4689-4693. doi: 10.3892/ol.2017.6733. Epub 2017 Aug 8. Oncol Lett. 2017. PMID: 28943963 Free PMC article. - Hematopoietic stem cell-derived adipocytes and fibroblasts in the tumor microenvironment.
Xiong Y, McDonald LT, Russell DL, Kelly RR, Wilson KR, Mehrotra M, Soloff AC, LaRue AC. Xiong Y, et al. World J Stem Cells. 2015 Mar 26;7(2):253-65. doi: 10.4252/wjsc.v7.i2.253. World J Stem Cells. 2015. PMID: 25815113 Free PMC article. Review.
References
- Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol. 2011;55:841–849. - PubMed
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. - PubMed
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous