Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex - PubMed (original) (raw)
Review
Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex
Santos J Franco et al. Neuron. 2013.
Abstract
The neural circuits of the mammalian neocortex are crucial for perception, complex thought, cognition, and consciousness. This circuitry is assembled from many different neuronal subtypes with divergent properties and functions. Here, we review recent studies that have begun to clarify the mechanisms of cell-type specification in the neocortex, focusing on the lineage relationships between neocortical progenitors and subclasses of excitatory projection neurons. These studies reveal an unanticipated diversity in the progenitor pool that requires a revised view of prevailing models of cell-type specification in the neocortex. We propose a "sequential progenitor-diversification model" that integrates current knowledge to explain how projection neuron diversity is achieved by mechanisms acting on proliferating progenitors and their postmitotic offspring. We discuss the implications of this model for our understanding of brain evolution and pathological states of the neocortex.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
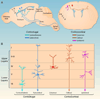
Figure 1. Major subtypes of neocortical projection neurons, classified by projection pattern and laminar position
(A) Sagittal view of corticofugal projection neurons in lower layers. Corticothalamic neurons are found mostly in layer VI and project to the thalamus. Subcerebral projection neurons are located in the lower parts of layer V and send primary projections to the spinal cord, pons and superior colliculus. (B) Coronal view of corticocortical projection neurons in upper layers. Most ipsilateral and callosal projection neurons are located in layers II-III and project within the same hemisphere or to the contralateral hemisphere via the corpus callosum, respectively. A subset of callosal neurons is also found in the superficial parts of layer V (not shown). Columnar projection neurons are found in layer IV and send short axons locally within a neocortical column. (C) Magnified view of projection neuron subtypes represented in A and B, detailing the relationships between laminar positions and projection patterns.
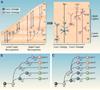
Figure 2. Different models of projection neuron cell-type specification in the developing neocortex
(A) A single kind of RGC progenitor sequentially generates all subtypes of projection neurons and macroglia. The fate potential of the common RGC is progressively restricted over time; thus, projection neuron subtype identity is specified by birth date. (B) Distinct subtypes of RGC progenitors co-exist and are pre-specified to generate different subtypes of projection neurons and macroglia. Projection neuron subtype fate is therefore specified by progenitor type, rather than by birth date. VZ, ventricular zone; SVZ, subventricular zone; LL, lower layers; UL, upper layers; MZ, marginal zone.
Figure 3. Subtypes of stem and progenitor cells in the developing neocortex
Early in neocortical development, neuroepithelial cells divide symmetrically to expand the progenitor pool before transforming into radial glial cells (RGCs). RGCs typically divide asymmetrically to self-renew and produce either neurons or intermediate progenitor cells (IPCs). IPCs divide symmetrically to generate pairs of neurons, or in some cases additional IPCs that then make neurons. Short neural precursors are similar to IPCs in that they undergo terminal symmetric divisions to make neurons, but like RGCs they maintain an apical end foot and are located in the ventricular zone. Basal RGCs (bRGCs) have a basal attachment at the pial basement membrane similar to RGCs, but do not maintain an apical process and thus have their cell bodies located in the outer margins of the SVZ. bRGCs self-renew (not shown) and generate IPCs and neurons. At the end of neurogenesis, RGCs and bRGCs transform into astrocyte progenitors. For abbreviations see legend to Fig. 2.
Figure 4. Distinct lineages of fate-restricted RGCs generate lower- versus upper-layer projection neurons
(A) Corticofugal projection neurons in lower layers V-VI are generated from Cux2− RGCs that are neurogenic during early stages of neocortical development. Corticocortical projection neurons in upper layers II-IV are made from Cux2+ RGCs that co-exist with Cux2− RGCs during early stages of neocortical development, but are initially proliferative and only become neurogenic at later developmental stages. (B-C) Hypothetical lineage trees for the different subtypes of neocortical projection neurons. In both models, neuroepithelial cells transform into RGCs, which are then specified into the Cux2+ and Cux2− lineages. In (B), Cux2+ and Cux2− RGCs are then progressively restricted over time to sequentially generate the different projection neuron subtypes based on birth date. In (C), progenitors of the two lineages undergo further specification at the progenitor state to generate additional lineages of fate-restricted RGCs that produce a specific projection neuron subtype. For abbreviations see legend to Fig. 2.
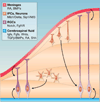
Figure 5. Signals that control RGC proliferative behavior
A number of signaling molecules that are secreted by the meninges or that are present in the cerebrospinal fluid can act on RGCs to regulate their proliferative and neurogenic behaviors. Similarly, IPCs and neurons secrete signals that provide a feedback mechanism for controlling the balance between proliferation and neurogenesis in RGCs.
Similar articles
- Molecular logic of neocortical projection neuron specification, development and diversity.
Greig LC, Woodworth MB, Galazo MJ, Padmanabhan H, Macklis JD. Greig LC, et al. Nat Rev Neurosci. 2013 Nov;14(11):755-69. doi: 10.1038/nrn3586. Epub 2013 Oct 9. Nat Rev Neurosci. 2013. PMID: 24105342 Free PMC article. Review. - Precision in the development of neocortical architecture: From progenitors to cortical networks.
Kast RJ, Levitt P. Kast RJ, et al. Prog Neurobiol. 2019 Apr;175:77-95. doi: 10.1016/j.pneurobio.2019.01.003. Epub 2019 Jan 21. Prog Neurobiol. 2019. PMID: 30677429 Free PMC article. Review. - Coupling progenitor and neuronal diversity in the developing neocortex.
Govindan S, Jabaudon D. Govindan S, et al. FEBS Lett. 2017 Dec;591(24):3960-3977. doi: 10.1002/1873-3468.12846. Epub 2017 Oct 16. FEBS Lett. 2017. PMID: 28895133 Review. - Neural progenitors, neurogenesis and the evolution of the neocortex.
Florio M, Huttner WB. Florio M, et al. Development. 2014 Jun;141(11):2182-94. doi: 10.1242/dev.090571. Development. 2014. PMID: 24866113 Review. - Post-transcriptional regulatory elements and spatiotemporal specification of neocortical stem cells and projection neurons.
DeBoer EM, Kraushar ML, Hart RP, Rasin MR. DeBoer EM, et al. Neuroscience. 2013 Sep 17;248:499-528. doi: 10.1016/j.neuroscience.2013.05.042. Epub 2013 May 30. Neuroscience. 2013. PMID: 23727006 Free PMC article. Review.
Cited by
- Rbm8a haploinsufficiency disrupts embryonic cortical development resulting in microcephaly.
Mao H, Pilaz LJ, McMahon JJ, Golzio C, Wu D, Shi L, Katsanis N, Silver DL. Mao H, et al. J Neurosci. 2015 May 6;35(18):7003-18. doi: 10.1523/JNEUROSCI.0018-15.2015. J Neurosci. 2015. PMID: 25948253 Free PMC article. - Defining modulatory inputs into CNS neuronal subclasses by functional pharmacological profiling.
Raghuraman S, Garcia AJ, Anderson TM, Twede VD, Curtice KJ, Chase K, Ramirez JM, Olivera BM, Teichert RW. Raghuraman S, et al. Proc Natl Acad Sci U S A. 2014 Apr 29;111(17):6449-54. doi: 10.1073/pnas.1404421111. Epub 2014 Apr 14. Proc Natl Acad Sci U S A. 2014. PMID: 24733934 Free PMC article. - Cadherins as regulators of neuronal polarity.
Gärtner A, Fornasiero EF, Dotti CG. Gärtner A, et al. Cell Adh Migr. 2015;9(3):175-82. doi: 10.4161/19336918.2014.983808. Epub 2014 Nov 14. Cell Adh Migr. 2015. PMID: 25482615 Free PMC article. Review. - Prox1 Regulates the Subtype-Specific Development of Caudal Ganglionic Eminence-Derived GABAergic Cortical Interneurons.
Miyoshi G, Young A, Petros T, Karayannis T, McKenzie Chang M, Lavado A, Iwano T, Nakajima M, Taniguchi H, Huang ZJ, Heintz N, Oliver G, Matsuzaki F, Machold RP, Fishell G. Miyoshi G, et al. J Neurosci. 2015 Sep 16;35(37):12869-89. doi: 10.1523/JNEUROSCI.1164-15.2015. J Neurosci. 2015. PMID: 26377473 Free PMC article. - Relief of hypoxia by angiogenesis promotes neural stem cell differentiation by targeting glycolysis.
Lange C, Turrero Garcia M, Decimo I, Bifari F, Eelen G, Quaegebeur A, Boon R, Zhao H, Boeckx B, Chang J, Wu C, Le Noble F, Lambrechts D, Dewerchin M, Kuo CJ, Huttner WB, Carmeliet P. Lange C, et al. EMBO J. 2016 May 2;35(9):924-41. doi: 10.15252/embj.201592372. Epub 2016 Feb 8. EMBO J. 2016. PMID: 26856890 Free PMC article.
References
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–679. - PubMed
- Anderson JC, Douglas RJ, Martin KA, Nelson JC. Synaptic output of physiologically identified spiny stellate neurons in cat visual cortex. J Comp Neurol. 1994;341:16–24. - PubMed
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
Publication types
MeSH terms
Grants and funding
- HD070494/HD/NICHD NIH HHS/United States
- U01 MH078833/MH/NIMH NIH HHS/United States
- MH078833/MH/NIMH NIH HHS/United States
- P01 HD070494/HD/NICHD NIH HHS/United States
- NS046456/NS/NINDS NIH HHS/United States
- F32 NS060355/NS/NINDS NIH HHS/United States
- R01 NS046456/NS/NINDS NIH HHS/United States
- NS060355/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources