In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells - PubMed (original) (raw)
In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells
Ulf Dettmer et al. J Biol Chem. 2013.
Abstract
Aggregation of α-synuclein (αSyn) in neurons produces the hallmark cytopathology of Parkinson disease and related synucleinopathies. Since its discovery, αSyn has been thought to exist normally in cells as an unfolded monomer. We recently reported that αSyn can instead exist in cells as a helically folded tetramer that resists aggregation and binds lipid vesicles more avidly than unfolded recombinant monomers (Bartels, T., Choi, J. G., and Selkoe, D. J. (2011) Nature 477, 107-110). However, a subsequent study again concluded that cellular αSyn is an unfolded monomer (Fauvet, B., Mbefo, M. K., Fares, M. B., Desobry, C., Michael, S., Ardah, M. T., Tsika, E., Coune, P., Prudent, M., Lion, N., Eliezer, D., Moore, D. J., Schneider, B., Aebischer, P., El-Agnaf, O. M., Masliah, E., and Lashuel, H. A. (2012) J. Biol. Chem. 287, 15345-15364). Here we describe a simple in vivo cross-linking method that reveals a major ~60-kDa form of endogenous αSyn (monomer, 14.5 kDa) in intact cells and smaller amounts of ~80- and ~100-kDa forms with the same isoelectric point as the 60-kDa species. Controls indicate that the apparent 60-kDa tetramer exists normally and does not arise from pathological aggregation. The pattern of a major 60-kDa and minor 80- and 100-kDa species plus variable amounts of free monomers occurs endogenously in primary neurons and erythroid cells as well as neuroblastoma cells overexpressing αSyn. A similar pattern occurs for the homologue, β-synuclein, which does not undergo pathogenic aggregation. Cell lysis destabilizes the apparent 60-kDa tetramer, leaving mostly free monomers and some 80-kDa oligomer. However, lysis at high protein concentrations allows partial recovery of the 60-kDa tetramer. Together with our prior findings, these data suggest that endogenous αSyn exists principally as a 60-kDa tetramer in living cells but is lysis-sensitive, making the study of natural αSyn challenging outside of intact cells.
Figures
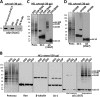
FIGURE 1.
HEL cells express high levels of endogenous αSyn that can be specifically cross-linked in vivo as a major ∼60-kDa species and minor species that include ∼80 and ∼100 kDa. A, cytosols (post-20,000 × g) from HEL cells, rat primary cortical neurons (DIV13), and M17D and SK-N-MC human neuroblastoma cells were immunoblotted with the αSyn mAb Syn1. Identical exposures of the same blot are shown; film was cut at the white line. B, HEL cells underwent in vivo cross-linking using the cell-permeant amine-reactive cross-linkers DSG, DSS, and DSP (each at 1 m
m
). Control cells (−) were treated with DMSO vehicle alone. Cytosols were generated by lysing cells via sonication for 15 s in PBS/PI and centrifuging at 20,000 × g. As controls for cross-linking efficiency, blots were stained with Ponceau solution or a rabbit pAb (10) to the dimeric cytosolic protein DJ-1. As negative controls, the monomeric GTPase Ran as well as β-tubulin were detected using specific antibodies. αSyn was detected using rat mAb 15G7; short and long exposures of the same blot are shown. C, HEL cells were subjected to in vivo cross-linking with the cleavable cross-linker DSP (1 m
m
), and cytosols were prepared (post-20,000 × g). For lanes labeled DSP/β_ME_ (β-mercaptoethanol), the DSP cross-linker was cleaved after cross-linking by boiling in sample buffer with 5% (v/v) β-mercaptoethanol, whereas lanes labeled DSP were boiled in sample buffer alone. General cross-linking efficiency was visualized by Ponceau (Ponc.) staining and by blotting for DJ-1. D, HEL cells underwent in vivo cross-linking using the short spacer length cross-linker DFDNB (1 m
m
) versus DMSO alone (−), and 20,000 × g cytosols were prepared. Ponceau staining and blotting for DJ-1 verified the general efficiency of the cross-linker.
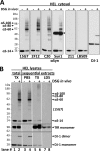
FIGURE 2.
Multiple αSyn antibodies confirm the specificity of the ∼60-, ∼80-, and ∼100-kDa species, all of which are enriched in the high speed cytosol fraction. A, HEL cells were treated in vivo with DMSO alone (−) or 1 m
m
DSG (+), sonicated in PBS/PI, and spun at 435,000 × g for 30 min. The resultant cytosols were blotted with αSyn antibodies 15G7, 2F12, C20, Syn1, 211, or LB509 and with DJ-1 antibody (10) as a control for cross-linking efficiency. Note that all six αSyn antibodies revealed lower levels of free monomer (14 kDa) after cross-linking. See “Results” for details. The asterisk marks a crossreactive band detected by the Syn1 antibody. B, shown are TX-100 total lysates and sequential extracts of HEL cells treated in vivo with DMSO (−) or 1 m
m
DSG (+). Total lysates (left panel) were generated by bringing cells lysed by sonication in PBS/PI to 1% TX-100 (TX) followed by 10 min incubation on ice and ultracentrifugation (435,000 × g, 30 min). For sequential extractions (right three panels), see “Experimental Procedures” and “Results.” Enrichment of soluble proteins in the PBS cytosol was confirmed by strong signals for the cytosolic protein DJ-1 (monomer and dimer) and the absence of transferrin receptor (TfR). The TX-100 fractions showed the opposite pattern, consistent with enrichment of membrane proteins. mAb 15G7 revealed co-fractionation of all αSyn species with DJ-1 in the cytosol; only minor αSyn amounts were detected in the TX and LDS fractions. Identical exposures of the same blot are shown; film was cut at the white lines.
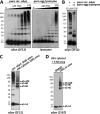
FIGURE 3.
αSyn 60-, 80-, and 100-kDa cross-linked species are apparent homo-oligomers having different conformations or monomer numbers. A, wt αSyn was transiently expressed in the M17D neuroblastoma cell line for 48 h followed by in vivo cross-linking. As a control, mock-transfected cells (-) are shown. B, αSyn was expressed in E. coli BL21 cells (11) in the presence (right panel, right three lanes; strong expression) or absence (right panel, left three lanes; weaker background expression) of the inducer IPTG. As a control, lysates from wt E. coli BL21 are shown in the left panel. DSG concentrations of 0, 0.3, and 1 m
m
were applied to intact bacterial cells. IPTG, isopropyl 1-thio-β-
d
-galactopyranoside. C, incomplete in vivo cross-linking of αSyn was analyzed by one-dimensional SDS-PAGE (one-dimensional (1D)) and two-dimensional gel-electrophoresis (2D). One-dimensional panels are on the left. SDS-PAGE/WB analysis is shown for cytosols from HEL cells DSG-cross-linked at 4 °C, resulting in an enhanced relative detection of monomers, putative αSyn dimers (α_S-30_), and trimers (α_S-40_). Two-dimensional panels on the right, control-treated as well as incompletely in vivo DSG-cross-linked HEL cell cytosols were run in a two-dimensional gel system. Proteins were separated by isoelectric focusing in the x axis at a pH range of 3.5–6.0 and in the y-axis by standard SDS-PAGE. In DMSO-treated samples (upper large panel and small panel showing a short exposure of the 14-kDa region), almost all αSyn focused at 14 kDa (y axis) and around its theoretical isoelectric point of pH 4.67 (x axis) as expected. Reaction of DSG with the αSyn species is expected to shift their isoelectric points to a more acidic position due to the masking of the positive charge of lysines. Therefore, in vivo DSG cross-linking (lower large panel and small panel show a short exposure of the 14-kDa region) yielded a 14-kDa spot at the position of unmodified monomers (compare with top panel) plus monomers at slightly more acidic positions (farther left), suggesting partial intramolecular modification of lysines in the monomer. A similar shift toward the lower pH range was observed for the αS-60, -80, and -100 species such that their isoelectric points aligned with the acid-shifted monomers. D, shown is the co-IP analysis of differently tagged αSyn molecules after co-expression and cross-linking. αSyn-mycHis and αSyn-FLAG3 were either expressed separately in two different cell populations and mixed just before in vivo cross-linking (M) or co-expressed in the same cell population and then mixed with mock-transfected cells just before in vivo cross-linking (C). After the in vivo cross-linking, lysates were subjected to FLAG-IP. Starting materials (lysate) and anti-FLAG IPs (IP M2) were analyzed by WB using specific antibodies for the myc-epitope (A14), the FLAG-epitope (M2), and αSyn (15G7). A short exposure of the monomer bands in the anti-myc blot demonstrates the relative depletion of monomers in comparison to the mid-_M_r species shown in the long exposure of the blot. IP purity was confirmed by the absence of DJ-1 immunoreactivity (left panel) in the IP lanes. The asterisk marks IgG light chain bands.
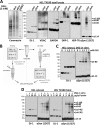
FIGURE 4.
αS-60, -80, and -100 are homo-oligomers distinct from the products of diffusion-controlled cross-linking. A, pure recombinant (rec.) αSyn and pure egg lysozyme were cross-linked at a concentration of 10 ng/μl using DSG concentrations of 0, 1, 3, 5, 10, 30, 100 μ
m
, leading to an increasing detection of induced oligomers by WB using specific antibodies. Of note, the induced αSyn oligomers ran higher than the respective lysozyme oligomers, presumably due to a different structure of the cross-linked species. B, pure recombinant αSyn at a concentration of 10 ng/μl was cross-linked using 30 μ
m
DSG in the presence (right panel) or absence (left panel) of a 10-fold molar excess of egg lysozyme. C, pure recombinant αSyn cross-linked at a concentration of 10 ng/μl using 30 μ
m
DSG (left lane) was run on an SDS-PAGE next to a cytosol of HEL cells that had been subjected to standard DSG in vivo cross-linking. D, HEL cells were treated with DSG or just DMSO in vivo, and cytosols were prepared; these were then treated with 5
m
urea (final concentration), boiled for 10 min, and run on SDS-PAGE.
FIGURE 5.
The interactions of αSyn revealed by in vivo cross-linking are sensitive to cell lysis. A, αS-80, but not αS-60 or αS-100, is relatively resistant to cell lysis. The occurrence of in vivo and in vitro protein cross-linking was confirmed by Coomassie-staining of SDS-PAGE gels (far left panel) as well as for αSyn (mAb 15G7) and certain other normally oligomeric proteins by blotting with the indicated antibodies: DRP-1, dynamin-related protein 1; HSP-70, heat shock 70 kDa protein; VDAC, voltage-dependent anion channel protein. HEL TX-100 total lysates (spun at 213,000 × g) were analyzed. _M_r, molecular weight marker (SeeBlue Plus2). B, a schematic is shown of in vivo and in vitro cross-linking protocols (see “Experimental Procedures” and “Results” for details). C, increasing DSG concentration does not overcome the inability to trap αSyn species other than αS-80 in vitro. 4 × 106 HEL cells in 200 μl PBS/PI were lysed by sonication, treated with 1, 1.5, 2, or 5 m
m
DSG and spun at 213,000 × g. D, in vitro cross-linking in lysates of high protein concentration partially recovers the in vivo αSyn cross-linking pattern by stabilizing αS-60. Concentrated (15 μg/μl) lysates were generated by lysing HEL cells in small volumes of either PBS/PI (two panels on the left) or PBS/PI/1% TX-100 (two panels on the right) followed by centrifugation at 213,000 × g; decreasing concentrations were generated by diluting this 15 μg/μl sample. After cross-linking at the indicated protein concentrations, all samples were normalized to 5 μg/μl, and equal volumes were loaded. Of note, oligomerization intermediates, the putative dimer αS-30, and the putative trimer αS-40 could be detected in some of the sample to variable degrees.
FIGURE 6.
Cross-linking of endogenous α- and β-synuclein in primary neurons and endogenous and transfected αSyn in M17D cells validates the findings in HEL cells. A, shown is an immunoblot analysis of cytosols (213,000_g_) after DSG cross-linking of rat neurons and M17D cells. Primary cortical rat neurons (DIV13) were cross-linked in vivo or in vitro just as with HEL cells. pAb to Sbr-2 (left panel) detected a strong signal in a total protein lysate (to.) but not in cytosols. Blotting for DJ-1 confirmed the equal efficiency of in vivo and in vitro cross-linking. pAb EP1537Y was used to probe for βSyn, and mAb 2F12 was used for αSyn. B, shown is an immunoblot analysis of 1% TX-100 total lysates (213,000 × g) after in vivo DSG cross-linking of primary cortical rat neurons (DIV13). For comparison, a cytosol (cy.) is shown. Unlike Sbr-2, which was absent in cytosol, αSyn was equally present in cytosolic (cy.) and TX-100 total lysates (total). A weak ∼35-kDa band (arrowhead) was detected for Sbr-2 after cross-linking. The asterisks marks the nonspecific band detected by the antibody (see also Fig. 2_B_). C, rat neurons (DIV13) were cross-linked with increasing concentrations of DSG (0–5 m
m
) and immediately lysed in 2% LDS sample buffer. The asterisk marks a presumably nonspecific band detected by the antibody in rat neuronal lysates; g.e., gel-excluded. D, Wt αSyn and the NAC-domain mutant Δ71–82 were transiently expressed in M17D cells for 48 h followed by in vivo cross-linking with DSG (right panels in each pair) or just DMSO (left panels in each pair). As a control, vector only was transfected (mock). DJ-1 was a control for cross-linking efficiency and equal loading (left panel). Identical exposures of the same blot are shown; film was cut at white lines.
Similar articles
- Defining the native state of α-synuclein.
Selkoe D, Dettmer U, Luth E, Kim N, Newman A, Bartels T. Selkoe D, et al. Neurodegener Dis. 2014;13(2-3):114-7. doi: 10.1159/000355516. Epub 2013 Oct 30. Neurodegener Dis. 2014. PMID: 24192542 Review. - Purification of α-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity.
Luth ES, Bartels T, Dettmer U, Kim NC, Selkoe DJ. Luth ES, et al. Biochemistry. 2015 Jan 20;54(2):279-92. doi: 10.1021/bi501188a. Epub 2014 Dec 23. Biochemistry. 2015. PMID: 25490121 Free PMC article. - α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation.
Bartels T, Choi JG, Selkoe DJ. Bartels T, et al. Nature. 2011 Aug 14;477(7362):107-10. doi: 10.1038/nature10324. Nature. 2011. PMID: 21841800 Free PMC article. - 14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein.
Wang B, Underwood R, Kamath A, Britain C, McFerrin MB, McLean PJ, Volpicelli-Daley LA, Whitaker RH, Placzek WJ, Becker K, Ma J, Yacoubian TA. Wang B, et al. J Neurosci. 2018 Sep 19;38(38):8211-8232. doi: 10.1523/JNEUROSCI.1134-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093536 Free PMC article. - Exploring the accessible conformations of N-terminal acetylated α-synuclein.
Moriarty GM, Janowska MK, Kang L, Baum J. Moriarty GM, et al. FEBS Lett. 2013 Apr 17;587(8):1128-38. doi: 10.1016/j.febslet.2013.02.049. Epub 2013 Mar 13. FEBS Lett. 2013. PMID: 23499431 Free PMC article. Review.
Cited by
- The Role of Lipids in the Initiation of α-Synuclein Misfolding.
Kiechle M, Grozdanov V, Danzer KM. Kiechle M, et al. Front Cell Dev Biol. 2020 Sep 15;8:562241. doi: 10.3389/fcell.2020.562241. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33042996 Free PMC article. Review. - Neurons and Glia Interplay in α-Synucleinopathies.
Mavroeidi P, Xilouri M. Mavroeidi P, et al. Int J Mol Sci. 2021 May 8;22(9):4994. doi: 10.3390/ijms22094994. Int J Mol Sci. 2021. PMID: 34066733 Free PMC article. Review. - How important is the N-terminal acetylation of alpha-synuclein for its function and aggregation into amyloids?
Iyer A, Sidhu A, Subramaniam V. Iyer A, et al. Front Neurosci. 2022 Nov 16;16:1003997. doi: 10.3389/fnins.2022.1003997. eCollection 2022. Front Neurosci. 2022. PMID: 36466161 Free PMC article. Review. - Parkinson-causing α-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation.
Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D. Dettmer U, et al. Nat Commun. 2015 Jun 16;6:7314. doi: 10.1038/ncomms8314. Nat Commun. 2015. PMID: 26076669 Free PMC article. - Female Sex and Brain-Selective Estrogen Benefit α-Synuclein Tetramerization and the PD-like Motor Syndrome in 3K Transgenic Mice.
Rajsombath MM, Nam AY, Ericsson M, Nuber S. Rajsombath MM, et al. J Neurosci. 2019 Sep 18;39(38):7628-7640. doi: 10.1523/JNEUROSCI.0313-19.2019. Epub 2019 Aug 12. J Neurosci. 2019. PMID: 31405930 Free PMC article.
References
- Fauvet B., Mbefo M. K., Fares M. B., Desobry C., Michael S., Ardah M. T., Tsika E., Coune P., Prudent M., Lion N., Eliezer D., Moore D. J., Schneider B., Aebischer P., El-Agnaf O. M., Masliah E., Lashuel H. A. (2012) α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and .Escherichia coli exists predominantly as disordered monomer., J. Biol. Chem. 287, 15345–15364 - PMC - PubMed
- Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 - PubMed
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 - PubMed
- Weinreb P. H., Zhen W., Poon A. W., Conway K. A., Lansbury P. T., Jr. (1996) NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35, 13709–13715 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous