Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy - PubMed (original) (raw)
Review
Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy
Srinivas Ramishetti et al. Ther Deliv. 2012 Dec.
Abstract
Nanotechnology is rapidly evolving and dramatically changing the paradigms of drug delivery. The small sizes, unique chemical properties, large surface areas, structural diversity and multifunctionality of nanoparticles prove to be greatly advantageous for combating notoriously therapeutically evasive diseases such as cancer. Multifunctional nanoparticles have been designed to enhance tumor uptake through either passive or active targeting, while also avoiding reticuloendothelial system uptake through the incorporation of PEG onto the surface. First-generation nanoparticle systems, such as liposomes, are good carriers for drugs and nucleic acid therapeutics, although they have some limitations. These lipid bilayers are now being utilized as excellent carriers for drug-loaded, solid core particles such as iron oxide, mesoporus silica and calcium phosphate. In this article, their design, as well as their multifunctional role in cancer therapy are discussed.
Figures
Figure 1
Multifunctional nanoparticle.
Figure 2. Passive and active targeting
(A) Nanoparticles enter the tumor environment via the enhanced permeability and retention effect; (B) target-specific uptake by tumor cells is facilitated by receptor-specific ligands on the surface of nanoparticles. Reproduced and modified with permission from [57] © American Chemical Society (2009).
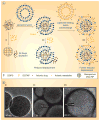
Figure 3. Supported bilayer coating of silica nanoparticles
(A) A negatively charged drug is adsorbed into the pores of a cationic mesoporous silica nanoparticle. (i) Other anions that are adsorbed more strongly can displace the loaded drugs. (ii) Fusion with a negatively charged liposome reduces the displacement, (iii) and further lipid exchange/fusion with cationic liposomes reduces it more. (B) Representative transmission electron microscopy images of (i) bare anionic mesoporous silica cores and protocells with (ii) single or (iii) dual supported bilayers formed after successive DOTAP and DOPS fusion/exchange steps (lipid-fixed and negative-stained). NP: Nanoparticle. Modified and reprinted with permission from [138] © American Chemical Society (2009).
Figure 4. Lipid bilayer supported multifunctional silica nanoparticle (protocell)
Reproduced with permission from [181] © American Chemical Society (2012).
Figure 5. Lipid-coated calcium phosphate nanoparticles
(A) Nontargeted and targeted LCP; (B) transmission electron microscopy images of LCP coated with DOTAP and DSPE–PEG. LCP: Lipid calcium phosphate. Reproduced with permission from [149] © American Chemical Society (2012).
Figure 6. Therapeutic effect of siRNA in liposomal calcium phosphate nanoparticles
(A) Photographs of lungs excised from tumor-bearing mice; (B) quantification of luciferase activity in lung metastasis; (C) survival analysis of B16F10 lung metastases bearing mice on day 19 after four treatments; and (D) intratumoral expression of VEGF after treatment. LCP: Lipid calcium phosphate; NP: Nanoparticle. Reproduced with permission from [151] © Macmillan Publishers Ltd. Molecular Therapy (2012).
Similar articles
- Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles.
Alavi M, Hamidi M. Alavi M, et al. Drug Metab Pers Ther. 2019 Feb 1;34(1). doi: 10.1515/dmpt-2018-0032. Drug Metab Pers Ther. 2019. PMID: 30707682 Review. - Designed Synthesis of Lipid-Coated Polyacrylic Acid/Calcium Phosphate Nanoparticles as Dual pH-Responsive Drug-Delivery Vehicles for Cancer Chemotherapy.
Wang X, Zhang M, Zhang L, Li L, Li S, Wang C, Su Z, Yuan Y, Pan W. Wang X, et al. Chemistry. 2017 May 11;23(27):6586-6595. doi: 10.1002/chem.201700060. Epub 2017 Apr 7. Chemistry. 2017. PMID: 28218434 - Role of integrated cancer nanomedicine in overcoming drug resistance.
Iyer AK, Singh A, Ganta S, Amiji MM. Iyer AK, et al. Adv Drug Deliv Rev. 2013 Nov;65(13-14):1784-802. doi: 10.1016/j.addr.2013.07.012. Epub 2013 Jul 21. Adv Drug Deliv Rev. 2013. PMID: 23880506 Review. - Progress in lipid-based nanoparticles for cancer therapy.
Grinberg S, Linder C, Heldman E. Grinberg S, et al. Crit Rev Oncog. 2014;19(3-4):247-60. doi: 10.1615/critrevoncog.2014011815. Crit Rev Oncog. 2014. PMID: 25271433 Review. - Targeted liposomal drug delivery: a nanoscience and biophysical perspective.
Liu Y, Castro Bravo KM, Liu J. Liu Y, et al. Nanoscale Horiz. 2021 Feb 1;6(2):78-94. doi: 10.1039/d0nh00605j. Epub 2021 Jan 5. Nanoscale Horiz. 2021. PMID: 33400747 Review.
Cited by
- Novel therapeutic mechanisms determine the effectiveness of lipid-core nanocapsules on melanoma models.
Drewes CC, Fiel LA, Bexiga CG, Asbahr AC, Uchiyama MK, Cogliati B, Araki K, Guterres SS, Pohlmann AR, Farsky SP. Drewes CC, et al. Int J Nanomedicine. 2016 Mar 31;11:1261-79. doi: 10.2147/IJN.S101543. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27099491 Free PMC article. - Enzyme-responsive nanomaterials for controlled drug delivery.
Hu Q, Katti PS, Gu Z. Hu Q, et al. Nanoscale. 2014 Nov 7;6(21):12273-86. doi: 10.1039/c4nr04249b. Nanoscale. 2014. PMID: 25251024 Free PMC article. Review. - Brain-targeted intranasal delivery of protein-based gene therapy for treatment of ischemic stroke.
Ryu JY, Cerecedo-Lopez C, Yang H, Ryu I, Du R. Ryu JY, et al. Theranostics. 2024 Aug 12;14(12):4773-4786. doi: 10.7150/thno.98088. eCollection 2024. Theranostics. 2024. PMID: 39239521 Free PMC article. - TIPS pentacene loaded PEO-PDLLA core-shell nanoparticles have similar cellular uptake dynamics in M1 and M2 macrophages and in corresponding in vivo microenvironments.
McDaniel DK, Jo A, Ringel-Scaia VM, Coutermarsh-Ott S, Rothschild DE, Powell MD, Zhang R, Long TE, Oestreich KJ, Riffle JS, Davis RM, Allen IC. McDaniel DK, et al. Nanomedicine. 2017 Apr;13(3):1255-1266. doi: 10.1016/j.nano.2016.12.015. Epub 2016 Dec 29. Nanomedicine. 2017. PMID: 28040495 Free PMC article. - Understanding the Nano-bio Interfaces: Lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications.
Luchini A, Vitiello G. Luchini A, et al. Front Chem. 2019 May 15;7:343. doi: 10.3389/fchem.2019.00343. eCollection 2019. Front Chem. 2019. PMID: 31165058 Free PMC article. Review.
References
- Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917. - PubMed
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. - PubMed
- Marshall E. Cancer research and the $90 billion metaphor. Science. 2011;331(6024):1540–1541. - PubMed
- Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA129421/CA/NCI NIH HHS/United States
- CA151652/CA/NCI NIH HHS/United States
- R01 CA149363/CA/NCI NIH HHS/United States
- CA129835/CA/NCI NIH HHS/United States
- U01 CA151455/CA/NCI NIH HHS/United States
- CA151455/CA/NCI NIH HHS/United States
- U54 CA151652/CA/NCI NIH HHS/United States
- R01 CA129835/CA/NCI NIH HHS/United States
- CA129421/CA/NCI NIH HHS/United States
- CA149363/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources