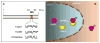FoxO6 in glucose metabolism (FoxO6) - PubMed (original) (raw)
Review
FoxO6 in glucose metabolism (FoxO6)
Dae Hyun Kim et al. J Diabetes. 2013 Sep.
Abstract
The forkhead box O (FoxO) subfamily has four members, namely FoxO1, FoxO3, FoxO4, and FoxO6. Unlike the other three members of the FoxO family, FoxO6 has garnered considerably less attention because of earlier reports that FoxO6 expression was limited to the brain. Recent data indicate that FoxO6 is produced in the liver of both rodents and humans. Hepatic FoxO6 activity, which remains at low basal levels in fed states, is markedly induced in fasted mice. FoxO6 activity becomes abnormally higher in the liver of mice with dietary obesity or type 2 diabetes (T2D). Genetically engineered mice with elevated FoxO6 activity in the liver exhibit prediabetes, culminating in the development of glucose intolerance, fasting hyperglycemia, and hyperinsulinemia. Conversely, inhibition of FoxO6 activity in the insulin-resistant liver results in a reduction in fasting hyperglycemia, contributing to the amelioration of hyperinsulinemia in T2D mice. These new data suggest that FoxO6 is an important regulator of hepatic glucose metabolism in response to insulin or physiological cues. Insulin inhibits FoxO6 activity by promoting its phosphorylation and disabling its activity in the nucleus without altering its subcellular distribution via a mechanism that is distinct from other members of the FoxO subfamily. In this article, we comprehensively review the role of FoxO6 in glucose metabolism in health and disease. We also address whether FoxO6 dysregulation is a contributing factor for the pathogenesis of fasting hyperglycemia and discuss whether FoxO6 is a potential therapeutic target for improving fasting hyperglycemia in T2D.
Keywords: FoxO6; FoxO6,葡萄糖异生,葡萄糖代谢,肝脏,2型糖尿病; gluconeognesis; glucose metabolism; liver; type 2 diabetes.
© 2013 Wiley Publishing Asia Pty Ltd and Ruijin Hospital, Shanghai Jiaotong University School of Medicine.
Conflict of interest statement
Disclosure:
None of the authors has a conflict of interest to declare in this manuscript
Figures
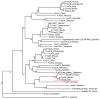
Fig. 1. Phylogeny of the FoxO subfamily
The phylogenetic tree for the FoxO subfamily was generated using the TreeFam program (
). The number in the phylogenic tree denotes the score that is linearly scaled from 1 to 100. The score measures how often the same ortholog (or paralog within-species) pair can be inferred from a resampled tree. The tree branches corresponding to the mammalian FoxO1 and FoxO6 are marked by red box.
Fig. 2. FoxO mediates insulin action on target gene expression
A. FoxO1 binds to the insulin response element (IRE) within the target promoter and promotes target gene expression in the absence of insulin. B. FoxO1 becomes phosphorylated in response to insulin. This effect promotes FoxO1 nuclear exclusion, resulting in the inhibition of target gene expression. N, nucleus. C, cytoplasm.
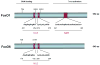
Fig. 3. Schematic depiction of mouse FoxO1 and FoxO6 proteins
FoxO1 and FoxO6 are characterized by the amino DNA binding motif and carboxyl _trans_-activation domain. They share a conserved bipartite basic nuclear localization signal (NLS) within the DNA binding domain. FoxO1 contains a nuclear export signal (NES) within its carboxyl _trans_-activation domain. Such a characteristic motif is lacking in FoxO6. FoxO1 contains three Akt/PKB phosphorylation sites (T24, S253, and S316). In contrast, only two Akt/PKB phosphorylation sites (T26 and S184) are present in FoxO6.
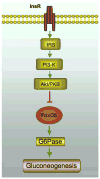
Fig. 4. FoxO6 mediates insulin action on gluconeogenesis
FoxO6 stimulates gluconeogenesis and this effect is counteracted by insulin. Insulin inhibits FoxO6 transcriptional activity by promoting FoxO6 phosphorylation, which in turn disables its cognate binding to the promoter of gluconeogenic genes (PEPCK and G6Pase) in liver. Insulin inhibition of FoxO6 activity in the liver is instrumental for limiting hepatic glucose production and preventing postprandial glucose excursion. In response to starvation, FoxO6 activity is upregulated, the resulting effect of which serves to prime the liver for augmented gluconeogenesis for maintaining fasting blood glucose levels within the physiological range. Unbridled FoxO6 activity, resulting from an impaired ability of insulin to keep FoxO6 action in check, promotes excessive gluconeogenesis in the liver and contributes to the episode of fasting hyperglycemia in diabetes. InsR, insulin receptor.
Fig. 5. A model of FoxO1 nucleocytoplasmic shuttling
A. FoxO1 contains a conserved NES within the trans-activation domain. In contrast, FoxO6 contains a semi-conserved NES. B. FoxO1 upon phosphorylation is associated with CRM-1, an exportin that is responsible for transporting FoxO1 from the nucleus to the cytoplasm.
Similar articles
- Forkhead Box O6 (FoxO6) Depletion Attenuates Hepatic Gluconeogenesis and Protects against Fat-induced Glucose Disorder in Mice.
Calabuig-Navarro V, Yamauchi J, Lee S, Zhang T, Liu YZ, Sadlek K, Coudriet GM, Piganelli JD, Jiang CL, Miller R, Lowe M, Harashima H, Dong HH. Calabuig-Navarro V, et al. J Biol Chem. 2015 Jun 19;290(25):15581-15594. doi: 10.1074/jbc.M115.650994. Epub 2015 May 5. J Biol Chem. 2015. PMID: 25944898 Free PMC article. - FoxO integration of insulin signaling with glucose and lipid metabolism.
Lee S, Dong HH. Lee S, et al. J Endocrinol. 2017 May;233(2):R67-R79. doi: 10.1530/JOE-17-0002. Epub 2017 Feb 17. J Endocrinol. 2017. PMID: 28213398 Free PMC article. Review. - FoxO6 integrates insulin signaling with gluconeogenesis in the liver.
Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, Fan Y, Giannoukakis N, Gramignoli R, Strom S, Ringquist S, Dong HH. Kim DH, et al. Diabetes. 2011 Nov;60(11):2763-74. doi: 10.2337/db11-0548. Epub 2011 Sep 22. Diabetes. 2011. PMID: 21940782 Free PMC article. - FoxO6 integrates insulin signaling with MTP for regulating VLDL production in the liver.
Kim DH, Zhang T, Lee S, Calabuig-Navarro V, Yamauchi J, Piccirillo A, Fan Y, Uppala R, Goetzman E, Dong HH. Kim DH, et al. Endocrinology. 2014 Apr;155(4):1255-67. doi: 10.1210/en.2013-1856. Epub 2014 Jan 17. Endocrinology. 2014. PMID: 24437489 Free PMC article. - Role of forkhead transcription factors in diabetes-induced oxidative stress.
Ponugoti B, Dong G, Graves DT. Ponugoti B, et al. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. Epub 2012 Jan 31. Exp Diabetes Res. 2012. PMID: 22454632 Free PMC article. Review.
Cited by
- Benzylglucosinolate Derived Isothiocyanate from Tropaeolum majus Reduces Gluconeogenic Gene and Protein Expression in Human Cells.
Guzmán-Pérez V, Bumke-Vogt C, Schreiner M, Mewis I, Borchert A, Pfeiffer AF. Guzmán-Pérez V, et al. PLoS One. 2016 Sep 13;11(9):e0162397. doi: 10.1371/journal.pone.0162397. eCollection 2016. PLoS One. 2016. PMID: 27622707 Free PMC article. - FoxO3a Serves as a Biomarker of Oxidative Stress in Human Lens Epithelial Cells under Conditions of Hyperglycemia.
Raju I, Kannan K, Abraham EC. Raju I, et al. PLoS One. 2013 Jun 21;8(6):e67126. doi: 10.1371/journal.pone.0067126. Print 2013. PLoS One. 2013. PMID: 23805295 Free PMC article. - Forkhead Box O6 (FoxO6) Depletion Attenuates Hepatic Gluconeogenesis and Protects against Fat-induced Glucose Disorder in Mice.
Calabuig-Navarro V, Yamauchi J, Lee S, Zhang T, Liu YZ, Sadlek K, Coudriet GM, Piganelli JD, Jiang CL, Miller R, Lowe M, Harashima H, Dong HH. Calabuig-Navarro V, et al. J Biol Chem. 2015 Jun 19;290(25):15581-15594. doi: 10.1074/jbc.M115.650994. Epub 2015 May 5. J Biol Chem. 2015. PMID: 25944898 Free PMC article. - Gluconeogenesis Alteration and p53-SIRT6-Fox01 Signaling Adaptive Regulation in Sheep from Different Grazing Periods.
Han Y, Liang C, Yu Y, Zhang J, Wang J, Cao J. Han Y, et al. Comput Math Methods Med. 2022 Jul 28;2022:4614665. doi: 10.1155/2022/4614665. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35936357 Free PMC article. Retracted. - Forkhead box family transcription factors as versatile regulators for cellular reprogramming to pluripotency.
Fu M, Chen H, Cai Z, Yang Y, Feng Z, Zeng M, Chen L, Qin Y, Cai B, Zhu P, Zhou C, Yu S, Guo J, Liu J, Cao S, Pei D. Fu M, et al. Cell Regen. 2021 Jul 2;10(1):17. doi: 10.1186/s13619-021-00078-4. Cell Regen. 2021. PMID: 34212295 Free PMC article.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. - PubMed
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. - PubMed
- Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644–647. - PubMed
- Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous