Toward the treatment and prevention of Alzheimer's disease: rational strategies and recent progress - PubMed (original) (raw)
Review
Toward the treatment and prevention of Alzheimer's disease: rational strategies and recent progress
Sam Gandy et al. Annu Rev Med. 2013.
Abstract
Alzheimer's disease (AD) is the major cause of late-life brain failure. In the past 25 years, autosomal dominant forms of AD were found to be primariy attributable to mutations in one of two presenilins, polytopic proteins that contain the catalytic site of the γ-secretase protease that releases the amyloid beta (Aβ) peptide. Some familial AD is also due to mutations in the amyloid precursor protein (APP), but recently a mutation in APP was discovered that reduces Aβ generation and is protective against AD, further implicating amyloid metabolism. Prion-like seeding of amyloid fibrils and neurofibrillary tangles has been invoked to explain the stereotypical spread of AD within the brain. Treatment trials with anti-Aβ antibodies have shown target engagement, if not significant treatment effects. Attention is increasingly focused on presymptomatic intervention, because Aβ mismetabolism begins up to 25 years before symptoms begin. AD trials deriving from new biological information involve extraordinary international collaboration and may hold the best hope for success in the fight against AD.
Figures
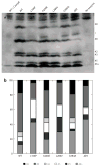
Figure 1
Polyacrylamide gel electrophoresis reveals a family of amyloid beta (Aβ) peptides generated by the processivity (trimming) function of γ-secretase following ε cleavage. (a) Effects of presenilin 1 (PS1) mutations (L166P, A246E, L286V, G384A, ΔE9) on γ-secretase processivity. Relative intensities of bands correspond to the differential generation of indicated species. (b) Schematized version of the data in panel a, illustrating the cleavage sites in the transmembrane domain of the amyloid precursor protein that specifies generation of each species. WT, wild type. Reprinted with permission from Reference .
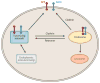
Figure 2
Schematized pathway showing the sorting of amyloid precursor protein (APP) (red dumbbell ) and BACE (β-secretase) (blue dumbbell ) into post-_trans_-Golgi network (TGN) compartments. The retromer retrieves endosomal proteins and conveys them to the TGN. Retromer deficiency leads to excess retention in the endosome and, theoretically, excess generation of Aβ because of enhanced cleavage of APP by BACE or (not shown) cleavage of APP carboxyl terminal fragments by γ-secretase. Reprinted with permission from Reference .
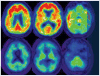
Figure 3
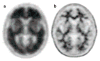
[11C] Pittsburgh compound B–based positron emission tomography reveals high fibrillar amyloid burden in the brain of a patient with Alzheimer’s disease (red signal, top row) as compared with the brain of an age-matched control subject (lower row). Reprinted with permission from Reference .
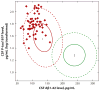
Figure 4
A plot of cerebrospinal fluid (CSF) levels of phospho-tau (P-tau) ( y axis) versus levels of amyloid beta (Aβ)1–42 reveals that all subjects in the cohort destined to develop Alzheimer’s disease (red diamonds in area 1) within 5 years showed a pattern of high CSF P-tau and low CSF Aβ1–42 and were excluded from areas of the plot defined by low CSF Aβ1–42 levels or low P-tau levels (defined by area 2 inside green circle). Reprinted with permission from Reference .
Figure 5
Amyvid® (18F-florbetapir)-based positron emission tomography images of the brains of (a) a subject with Alzheimer’s disease and (b) an age-matched control. The intense signal from the cerebral cortex indicates the presence of a heavy burden of fibrillar amyloid. Reprinted from
with the permission of Eli Lilly and Company.
Figure 6
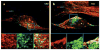
Differentiation of skin fibroblasts (UND, panel a) into neurons with the biochemical and physiological properties of cerebral cortical neurons reveals an apparent anomaly in endosomal morphology in the neurons from a subject with familial Alzheimer’s disease due to a presenilin 1 mutation. Differentiation of neurons (n) from the same subject (FAD, panel b) caused an exaggerated amyloid beta (Aβ)42/40 as compared with either the undifferentiated fibroblasts ( f ) or with Aβ42/40 generated by neurons differentiated from a control subject (not shown). Reprinted with permission from Reference . APP, amyloid precursor protein; MPR, mannose-6-phosphate receptor.
Figure 7
Anti–amyloid beta (Aβ) immunocytochemistry reveals acute deposition in amputated temporal lobes (a) at 2 h or (b) at 16 h following severe traumatic brain injury. Reprinted with permission from Reference .
Similar articles
- Autosomal-dominant Alzheimer's disease mutations at the same codon of amyloid precursor protein differentially alter Aβ production.
Suárez-Calvet M, Belbin O, Pera M, Badiola N, Magrané J, Guardia-Laguarta C, Muñoz L, Colom-Cadena M, Clarimón J, Lleó A. Suárez-Calvet M, et al. J Neurochem. 2014 Jan;128(2):330-9. doi: 10.1111/jnc.12466. Epub 2013 Oct 24. J Neurochem. 2014. PMID: 24117942 - Twenty Years of Presenilins--Important Proteins in Health and Disease.
Walter J. Walter J. Mol Med. 2015 Oct 27;21 Suppl 1(Suppl 1):S41-8. doi: 10.2119/molmed.2015.00163. Mol Med. 2015. PMID: 26605647 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review. - Regulation of Alzheimer beta-amyloid precursor trafficking and metabolism.
Gandy S, Petanceska S. Gandy S, et al. Biochim Biophys Acta. 2000 Jul 26;1502(1):44-52. doi: 10.1016/s0925-4439(00)00031-4. Biochim Biophys Acta. 2000. PMID: 10899430 Review. - Genes and mechanisms involved in beta-amyloid generation and Alzheimer's disease.
Steiner H, Capell A, Leimer U, Haass C. Steiner H, et al. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):266-70. doi: 10.1007/s004060050098. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10653281 Review.
Cited by
- Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers.
DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. DeKosky ST, et al. Nat Rev Neurol. 2013 Apr;9(4):192-200. doi: 10.1038/nrneurol.2013.36. Nat Rev Neurol. 2013. PMID: 23558985 Free PMC article. Review. - New Multifunctional Agents for Potential Alzheimer's Disease Treatment Based on Tacrine Conjugates with 2-Arylhydrazinylidene-1,3-Diketones.
Elkina NA, Grishchenko MV, Shchegolkov EV, Makhaeva GF, Kovaleva NV, Rudakova EV, Boltneva NP, Lushchekina SV, Astakhova TY, Radchenko EV, Palyulin VA, Zhilina EF, Perminova AN, Lapshin LS, Burgart YV, Saloutin VI, Richardson RJ. Elkina NA, et al. Biomolecules. 2022 Oct 24;12(11):1551. doi: 10.3390/biom12111551. Biomolecules. 2022. PMID: 36358901 Free PMC article. - Modeling autism by SHANK gene mutations in mice.
Jiang YH, Ehlers MD. Jiang YH, et al. Neuron. 2013 Apr 10;78(1):8-27. doi: 10.1016/j.neuron.2013.03.016. Neuron. 2013. PMID: 23583105 Free PMC article. Review. - Cognitive variability-A marker for incident MCI and AD: An analysis for the Alzheimer's Disease Neuroimaging Initiative.
Anderson ED, Wahoske M, Huber M, Norton D, Li Z, Koscik RL, Umucu E, Johnson SC, Jones J, Asthana S, Gleason CE; Alzheimer's Disease Neuroimaging Initiative. Anderson ED, et al. Alzheimers Dement (Amst). 2016 May 26;4:47-55. doi: 10.1016/j.dadm.2016.05.003. eCollection 2016. Alzheimers Dement (Amst). 2016. PMID: 27489880 Free PMC article. - Critical periods in adult neurogenesis and possible clinical utilization of new neurons.
Yamaguchi M, Mori K. Yamaguchi M, et al. Front Neurosci. 2014 Jun 24;8:177. doi: 10.3389/fnins.2014.00177. eCollection 2014. Front Neurosci. 2014. PMID: 25009460 Free PMC article. No abstract available.
References
- Bowen DM, Smith CB, Davison AN. Molecular changes in senile dementia. Brain. 1973;96:849–56. - PubMed
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. - PubMed
- Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts) N Engl J Med. 1980;302:1333–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX000348/BX/BLRD VA/United States
- U19 AG010483/AG/NIA NIH HHS/United States
- AG025204/AG/NIA NIH HHS/United States
- AG05133/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- R01 NS075685/NS/NINDS NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- P50 AG05138/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous