Neutralizing tumor-promoting chronic inflammation: a magic bullet? - PubMed (original) (raw)
Review
Neutralizing tumor-promoting chronic inflammation: a magic bullet?
Lisa M Coussens et al. Science. 2013.
Erratum in
- Science. 2013 Mar 29;339(6127):1522
Abstract
There have been substantial advances in cancer diagnostics and therapies in the past decade. Besides chemotherapeutic agents and radiation therapy, approaches now include targeting cancer cell-intrinsic mediators linked to genetic aberrations in cancer cells, in addition to cancer cell-extrinsic pathways, especially those regulating vascular programming of solid tumors. More recently, immunotherapeutics have entered the clinic largely on the basis of the recognition that several immune cell subsets, when chronically activated, foster tumor development. Here, we discuss clinical and experimental studies delineating protumorigenic roles for immune cell subsets that are players in cancer-associated inflammation. Some of these cells can be targeted to reprogram their function, leading to resolution, or at least neutralization, of cancer-promoting chronic inflammation, thereby facilitating cancer rejection.
Figures
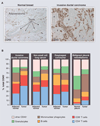
Fig. 1
Leukocyte infiltration and complexity in human cancers. (A) CD45+ leukocytes (brown staining) in normal human breast tissue compared with invasive ductal carcinoma. These images illustrate the substantial infiltration of leukocytes into neoplastic tissue compared with “normal” tissue counterparts. T indicates tumor nests or tumor cell clusters. (B) Immune cell complexity of adjacent normal tissues (or normal pleura) and the indicated tumors as revealed by polychromatic flow cytometry and expressed as a percentage of CD45+ cells. Colors indicate major categories of select immune cell lineages. [Images and data have not been published previously and are courtesy of the Coussens laboratory]
Fig. 2
Induction of TH2-type immune responses downstream of TSLP. DCs in tumor microenvironments are exposed to cancer-derived factors—for example, TSLP—that skew their maturation toward TH2-type inflammation, including their expression of OX40L. In this environment, responding TH2 cells (CD4+ T cells) secreting IL-4 and IL-13 promote tumor development either directly or indirectly via macrophages. Direct effects include triggering anti-apoptotic pathways and steroid metabolism in epithelial cancer cells, as well as promoting stromal fibroblast proliferation and differentiation. Indirect effects include triggering secretion of growth (EGF) and pro-angiogenic (VEGF) factors by tumor-infiltrating macrophages that also express inducible nitric oxide synthase (iNOS) and arginase (73) and thereby blunt CD8+ T cell proliferation.
Fig. 3
Therapeutic strategies against cancer-induced chronic inflammation. Inhibiting tumor cell–intrinsic proinflammatory functions [such as blunting NF-κB/STAT3/phosphatidylinositol 3-kinase (PI3K)–Akt pathways or downstream effectors]. Moreover, turning lymphocytes into effector TH1/TC1 cells necessitates effective reprogramming of type 2 macrophages or immunosuppressive DCs by a concerted action of pattern recognition receptors, the inflammasome platform, or CD40 costimulation, as well as neutralization of immune checkpoint ligand/receptor interaction. In parallel, reducing the accumulation or migration of suppressive myeloid cells in primary sites or distant niches while promoting cytoreduction/debulking with irradiation, cytotoxic compounds, or antiangiogenic molecules may synergistically gear the host/tumor imbalance toward durable tumor regression. HIF-1, hypoxia-inducible factor 1; AMPK, adenosine monophosphate–activated protein kinase; JAK2, Janus kinase 2; CDDO, 2-cyano- 3,12-dioxooleana-1,9(11)-dien-28-oic acid; TLR7, Toll-like receptor 7; COX2, cyclooxygenase; ICD, immunogenic cell death.
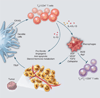
Fig. 4
Targeting tumor-promoting chronic inflammation as a therapeutic strategy. (A) Tissue damage results in activation of hard-wired pathways (angiogenic and immune) embedded in all tissues to facilitate healing and homeostasis. (B) Type 1 immune responses, aided by TH1 cells, eradicate damaged cells to aid the healing prcess. (C) In tissues harboring initiated cells, neoplastic epithelial cells secrete factors such as TSLP, GM-CSF, CSF-1, and TNFα, thereby inducing recruitment of leukocytes that become TH2-polarized and resulting in chronic activation of angiogenic and tissue remodeling programs, enhanced survival signaling to aid proliferation and blunt cell death, and generation of an immunosuppressive environment that fosters primary tumor development and aids in metastatic disseminations. (D) Effectively counter acting or neutralizing tumor-promoting chronic inflammation may be achieved by resetting or reprogramming the prominent TH2-based programs activated in cancer; this may result in simultaneously favoring (immunogenic) tumor cell death, where TH1-based immunity emerges akin to that present during acute inflammation during wound healing, thus enabling a cascade of events favoring cancer rejection, perhaps as monotherapy but more likely in combination with chemotherapy (CTX), radiotherapy (RT), targeted therapy (TT), or antiangiogenic modalities (αANG). DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
Similar articles
- Targeting cancer-related inflammation in the era of immunotherapy.
Nakamura K, Smyth MJ. Nakamura K, et al. Immunol Cell Biol. 2017 Apr;95(4):325-332. doi: 10.1038/icb.2016.126. Epub 2017 Jan 10. Immunol Cell Biol. 2017. PMID: 27999432 Review. - New insights into chronic inflammation-induced immunosuppression.
Kanterman J, Sade-Feldman M, Baniyash M. Kanterman J, et al. Semin Cancer Biol. 2012 Aug;22(4):307-18. doi: 10.1016/j.semcancer.2012.02.008. Epub 2012 Feb 24. Semin Cancer Biol. 2012. PMID: 22387003 Review. - Inflammation and cancer.
Coussens LM, Werb Z. Coussens LM, et al. Nature. 2002 Dec 19-26;420(6917):860-7. doi: 10.1038/nature01322. Nature. 2002. PMID: 12490959 Free PMC article. Review. - Overcoming Therapeutic Resistance by Targeting Cancer Inflammation.
Beatty GL. Beatty GL. Am Soc Clin Oncol Educ Book. 2016;35:e168-73. doi: 10.1200/EDBK_158948. Am Soc Clin Oncol Educ Book. 2016. PMID: 27249720 Review. - Myeloid-Derived Suppressor Cells and Proinflammatory Cytokines as Targets for Cancer Therapy.
Atretkhany KN, Drutskaya MS. Atretkhany KN, et al. Biochemistry (Mosc). 2016 Nov;81(11):1274-1283. doi: 10.1134/S0006297916110055. Biochemistry (Mosc). 2016. PMID: 27914453 Review.
Cited by
- Inhibition of Notch enhances efficacy of immune checkpoint blockade in triple-negative breast cancer.
Shen Q, Murakami K, Sotov V, Butler M, Ohashi PS, Reedijk M. Shen Q, et al. Sci Adv. 2024 Nov;10(44):eado8275. doi: 10.1126/sciadv.ado8275. Epub 2024 Oct 30. Sci Adv. 2024. PMID: 39475614 Free PMC article. - Myeloid Cell-associated Resistance to PD-1/PD-L1 Blockade in Urothelial Cancer Revealed Through Bulk and Single-cell RNA Sequencing.
Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, Farkas AM, Gnjatic S, Hake A, Izadmehr S, Wiklund P, Oh WK, Szabo PM, Wind-Rotolo M, Unsal-Kacmaz K, Yao X, Schadt E, Sharma P, Bhardwaj N, Zhu J, Galsky MD. Wang L, et al. Clin Cancer Res. 2021 Aug 1;27(15):4287-4300. doi: 10.1158/1078-0432.CCR-20-4574. Epub 2021 Apr 9. Clin Cancer Res. 2021. PMID: 33837006 Free PMC article. - Immune-modulating Effect of Korean Red Ginseng by Balancing the Ratio of Peripheral T Lymphocytes in Bile Duct or Pancreatic Cancer Patients With Adjuvant Chemotherapy.
Kim IK, Lee KY, Kang J, Park JS, Jeong J. Kim IK, et al. In Vivo. 2021 May-Jun;35(3):1895-1900. doi: 10.21873/invivo.12454. In Vivo. 2021. PMID: 33910879 Free PMC article. Clinical Trial. - Tumour-associated macrophages: versatile players in the tumour microenvironment.
Ji ZZ, Chan MK, Chan AS, Leung KT, Jiang X, To KF, Wu Y, Tang PM. Ji ZZ, et al. Front Cell Dev Biol. 2023 Oct 26;11:1261749. doi: 10.3389/fcell.2023.1261749. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37965573 Free PMC article. Review. - Tumor necrosis factor is dispensable for the success of immunogenic anticancer chemotherapy.
Ma Y, Yamazaki T, Yang H, Kepp O, Galluzzi L, Zitvogel L, Smyth MJ, Kroemer G. Ma Y, et al. Oncoimmunology. 2013 Jun 1;2(6):e24786. doi: 10.4161/onci.24786. Epub 2013 Apr 30. Oncoimmunology. 2013. PMID: 23894723 Free PMC article.
References
- Hanahan D, Weinberg RA. Cell. 2011;144:646. - PubMed
- Hanahan D, Coussens LM. Cancer Cell. 2012;21:309. - PubMed
- Balkwill F, Mantovani A. Lancet. 2001;357:539. - PubMed
- Bickels J, Kollender Y, Merinsky O, Meller I. Isr. Med. Assoc. J. 2002;4:471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous