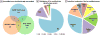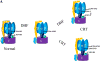Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system - PubMed (original) (raw)
Review
Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system
Heaseung S Chung et al. Circ Res. 2013.
Abstract
In the cardiovascular system, changes in oxidative balance can affect many aspects of cellular physiology through redox-signaling. Depending on the magnitude, fluctuations in the cell's production of reactive oxygen and nitrogen species can regulate normal metabolic processes, activate protective mechanisms, or be cytotoxic. Reactive oxygen and nitrogen species can have many effects including the posttranslational modification of proteins at critical cysteine thiols. A subset can act as redox-switches, which elicit functional effects in response to changes in oxidative state. Although the general concepts of redox-signaling have been established, the identity and function of many regulatory switches remains unclear. Characterizing the effects of individual modifications is the key to understand how the cell interprets oxidative signals under physiological and pathological conditions. Here, we review the various cysteine oxidative posttranslational modifications and their ability to function as redox-switches that regulate the cell's response to oxidative stimuli. In addition, we discuss how these modifications have the potential to influence other posttranslational modifications' signaling pathways though cross-talk. Finally, we review the increasing number of tools being developed to identify and quantify the various cysteine oxidative posttranslational modifications and how this will advance our understanding of redox-regulation.
Figures
Figure 1. Ox-PTMs on cysteine
A, Protective mode (Blue), from free thiol, modifications induced by small molecules: sulfhydration, S- nitrosylaion, S-glutahionylation (on bottom) and sulfenylation (in the middle). From sulfenic acid to reversible modifications: disulfide bond formation, sulfenylamide and S-glutathionylation. B, Non-protective/detrimental mode (red), irreversible modification: sulfinic acid and sulfonic acid as ROS level increases (bar on bottom). *: It has long been regarded this modification is irreversible but recently, there have been examples of enzymatic reduction including sulfiredoxin (Srx), which can reduce sulfinic acid in a subset of Peroxiredoxins (Prxs).–
Figure 2. Overview of the SNO modified Cys residues
A, The distribution of the 359 modified sites identified based on different experimental protocols is described in the text. B, Distribution of modified sites identified per protein. C, The subcellular localization of modified proteins as determined by annotation in the Uniprot database.
Figure 3. Redox sensor proteins and redox switches
A, ATP synthase α subunit Cys 294 functions as a redox switch. In dyssynchronous heart failure (DHF), Cys294 of the α-subunit are _S_-glutathionylated, or inter-disulfide bonding occurs between Cys294 of alpha-subunits, as well as between Cys294 and Cys103 of the gamma-subunit. Cys cross-linking inhibits its ATP production, leading to mitochondrial dysfunction. Cardiac resynchronization therapy (CRT) increases _S_-nitrosylation of ATP synthase at Cys294 of the alpha-subunit by reverse Cys cross-linking, along with recovered ATPase activity. B, Regulation of actin polymerization through S-glutathionylation of Cys374 of actin.
Figure 3. Redox sensor proteins and redox switches
A, ATP synthase α subunit Cys 294 functions as a redox switch. In dyssynchronous heart failure (DHF), Cys294 of the α-subunit are _S_-glutathionylated, or inter-disulfide bonding occurs between Cys294 of alpha-subunits, as well as between Cys294 and Cys103 of the gamma-subunit. Cys cross-linking inhibits its ATP production, leading to mitochondrial dysfunction. Cardiac resynchronization therapy (CRT) increases _S_-nitrosylation of ATP synthase at Cys294 of the alpha-subunit by reverse Cys cross-linking, along with recovered ATPase activity. B, Regulation of actin polymerization through S-glutathionylation of Cys374 of actin.
Figure 4. Proposed schemes for indirect (A and B) and direct (C and D) regulation by Ox-PTM of other PTMs and, vice versa
A, Indirect regulation by Ox-PTM of other PTM (ex.phosphorylation).B, Indirect regulation by other PTM (ex.glycation) of Ox-PTM (proposed mechanism). C and D, Direct regulation (cross-talk) between Ox-PTM and other PTMs in the same protein (proposed mechanism).
Similar articles
- Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation.
Mieyal JJ, Chock PB. Mieyal JJ, et al. Antioxid Redox Signal. 2012 Mar 15;16(6):471-5. doi: 10.1089/ars.2011.4454. Epub 2012 Jan 4. Antioxid Redox Signal. 2012. PMID: 22136616 Free PMC article. - Oxidative Post-Translational Modifications: A Focus on Cysteine _S-_Sulfhydration and the Regulation of Endothelial Fitness.
Bibli SI, Fleming I. Bibli SI, et al. Antioxid Redox Signal. 2021 Dec 20;35(18):1494-1514. doi: 10.1089/ars.2021.0162. Epub 2021 Sep 29. Antioxid Redox Signal. 2021. PMID: 34346251 - A direct way of redox sensing.
Benoit R, Auer M. Benoit R, et al. RNA Biol. 2011 Jan-Feb;8(1):18-23. doi: 10.4161/rna.8.1.13555. Epub 2011 Jan 1. RNA Biol. 2011. PMID: 21220941 - Redox proteomics: from bench to bedside.
Ckless K. Ckless K. Adv Exp Med Biol. 2014;806:301-17. doi: 10.1007/978-3-319-06068-2_13. Adv Exp Med Biol. 2014. PMID: 24952188 Review. - Pathological Impact of Redox Post-Translational Modifications.
Chahla C, Kovacic H, Ferhat L, Leloup L. Chahla C, et al. Antioxid Redox Signal. 2024 Jul;41(1-3):152-180. doi: 10.1089/ars.2023.0252. Epub 2024 Apr 8. Antioxid Redox Signal. 2024. PMID: 38504589 Review.
Cited by
- Post-translational Modifications in Brain Diseases: A Future for Biomarkers.
Silva-Costa LC, Smith BJ. Silva-Costa LC, et al. Adv Exp Med Biol. 2022;1382:129-141. doi: 10.1007/978-3-031-05460-0_10. Adv Exp Med Biol. 2022. PMID: 36029409 - Renal dopaminergic system: Pathophysiological implications and clinical perspectives.
Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, Fernández BE. Choi MR, et al. World J Nephrol. 2015 May 6;4(2):196-212. doi: 10.5527/wjn.v4.i2.196. World J Nephrol. 2015. PMID: 25949933 Free PMC article. Review. - Irreversible oxidative post-translational modifications in heart disease.
Tomin T, Schittmayer M, Honeder S, Heininger C, Birner-Gruenberger R. Tomin T, et al. Expert Rev Proteomics. 2019 Aug;16(8):681-693. doi: 10.1080/14789450.2019.1645602. Epub 2019 Jul 30. Expert Rev Proteomics. 2019. PMID: 31361162 Free PMC article. Review. - Production of adeno-associated virus vectors for in vitro and in vivo applications.
Kimura T, Ferran B, Tsukahara Y, Shang Q, Desai S, Fedoce A, Pimentel DR, Luptak I, Adachi T, Ido Y, Matsui R, Bachschmid MM. Kimura T, et al. Sci Rep. 2019 Sep 19;9(1):13601. doi: 10.1038/s41598-019-49624-w. Sci Rep. 2019. PMID: 31537820 Free PMC article. - Covalent Chemical Tools for Profiling Post-Translational Modifications.
Emenike B, Nwajiobi O, Raj M. Emenike B, et al. Front Chem. 2022 Jul 4;10:868773. doi: 10.3389/fchem.2022.868773. eCollection 2022. Front Chem. 2022. PMID: 35860626 Free PMC article. Review.
References
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. - PubMed
- Reddie KG, Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–54. - PubMed
- Roos G, Messens J. Protein sulfenic acid formation: from cellular damage to redox regulation. Free Radic Biol Med. 2011;51:314–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL081427/HL/NHLBI NIH HHS/United States
- N01HV28180/HV/NHLBI NIH HHS/United States
- P01HL77189-01/HL/NHLBI NIH HHS/United States
- R01 HL101235/HL/NHLBI NIH HHS/United States
- R21 HL112586/HL/NHLBI NIH HHS/United States
- HHSN268201000032C/HL/NHLBI NIH HHS/United States
- 1R01HL101235-01/HL/NHLBI NIH HHS/United States
- P01 HL077180/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources