Conserved spatial organization of FG domains in the nuclear pore complex - PubMed (original) (raw)
Conserved spatial organization of FG domains in the nuclear pore complex
Claire E Atkinson et al. Biophys J. 2013.
Abstract
Selective transport through the nuclear pore complex (NPC) requires nucleoporins containing natively unfolded phenylalanine-glycine (FG) domains. Several differing models for their dynamics within the pore have been proposed. We characterize the behavior of the FG nucleoporins in vivo using polarized fluorescence microscopy. Using nucleoporins tagged with green fluorescent protein along their FG domains, we show that some of these proteins are ordered, indicating an overall orientational organization within the NPC. This orientational ordering of the FG domains depends on their specific context within the NPC, but is independent of active transport and cargo load. For most nups, behavior does not depend on the FG motifs. These data support a model whereby local geometry constrains the orientational organization of the FG nups. Intriguingly, homologous yeast and mammalian proteins show conserved behavior, suggesting functional relevance. Our findings have implications for mechanistic models of NPC transport.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
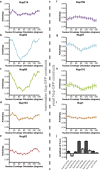
Figure 1
Anisotropy of FG nups with GFP at the tip. Anisotropy is plotted as a function of NE orientation. A modulated curve is indicative of a more ordered GFP; a higher amplitude (_r_0°–_r_90°) means more order. Homologous proteins are shown side-by-side. (a) mNup214-GFPtip, n = 34 cells. (b) mNup54-GFPtip, n = 39 cells. (c) mNup98-GFPtip, n = 32 cells. (d) mNup153-GFPtip, n = 32 cells. (e) mNup62-GFPtip, n = 46 cells. (f) yNup159-GFPtip, n = 123 cells. (g) yNup57-GFP-tip, n = 78 cells. (h) yNup116-GFPtip, n = 87 cells. (i) yNup1-GFPtip, n = 207 cells. (j) Amplitudes (_r_0°–_r_90°) of mammalian (dark-shaded) and yeast (light-shaded) anisotropy curves. All plots are mean ± SE.
Figure 2
Anisotropy of Nup-GFP constructs with GFP placed at different positions along the FG domain. (a) Schematic of GFP placement relative to the FG domain. (b and c) Amplitude (_r_0°–_r_90°) of anisotropy curves: (b) Yeast and (c) mammalian constructs. Numbers of cells analyzed are shown above each bar. Homologous proteins share the same shade. Numbers of cells analyzed is shown above each bar. (d_–_j) Example of proteins increasing in order from tip to base of the FG domain for Yeast Nup116: (d) yNup116-GFPtip; (e) yNup116-GFPmiddle; and (f) yNup116-GFPboundary. For Mammalian Nup54: (g) mNup54-GFPtip; (h) mNup54-GFPmiddle; (i) mNup54-GFPboundary; and (j) mNup54-GFPfolded. All plots mean ± SE.
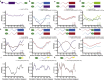
Figure 3
Moving the FG domains to a different position within the NPC causes changes in order. Anisotropy is plotted as a function of NE orientation. Schematics of the constructs are shown above graphs. (Top row) Central FG nup domains joined to the cytoplasmic Nup214 folded domain. (a) Native behavior of mNup214-GFPtip at the cytoplasmic face (purple). (b) Wild-type mNup54-GFPtip in center (blue); chimeric mNup214 coiled-coil/mNup54FG-GFPtip at the cytoplasmic face (light blue). (c) Wild-type mNup62-GFPtip in center (maroon); chimeric mNup214 coiled-coil/mNup62FG-GFPtip at the cytoplasmic face (red). (d) Wild-type mNup98-GFPtip in center (green); chimeric mNup214 coiled-coil/mNup98FG-GFPtip at the cytoplasmic face (dark green). (Middle row) Swapping FG and folded domains within a central subcomplex. (e) Native behaviors of mNup54-GFPtip (blue); mNup62-GFPtip (maroon). (f) Chimeras: mNup54 coiled-coil/mNup62FG-GFPtip (light blue); mNup62 coiled-coil/mNup54FG-GFPtip (red). (g) Native behaviors of mNup54-GFPboundary (blue); mNup62-GFPboundary (maroon). (h) Chimeras: mNup54 coiled-coil/mNup62FG-GFPboundary (light blue); mNup62 coiled-coil/mNup54FG-GFPboundary (red). (Bottom row) FG domains anchored at the plasma membrane by a palmitoyl moiety (yellow). (i) GFP-palmitoyl (black). (j) GFP-Nup54FG-palmitoyl (blue). (k) GFP-Nup62FG-palmitoyl (maroon). All plots are mean ± SE. The number of cells analyzed is indicated on each plot.
Figure 4
Contribution of FG repeats to ordering. Anisotropy is plotted as a function of NE orientation for wild-type and mutant FG→AG constructs. (a) mNup62-GFP constructs (top to bottom): tip, middle, boundary, folded; WT (maroon, gray in print) and FG→AG mutant (red, gray in print). (b) mNup54-GFP constructs (top to bottom): tip, middle, boundary, folded; WT (blue, gray in print) and FG→AG mutant (dark blue, dark gray in print). All plots are mean ± SE. The number of cells analyzed is indicated on each plot.
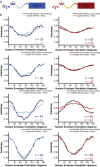
Figure 5
Ligand binding can be detected in anisotropy measurements. (a) Schematic of mNup54 (left) and mNup62 (right) with putative glycosylated regions (yellow diamonds). Position of the FG repeats relative to the glycosylated region is shown. (b and c) Anisotropy plotted against NE orientation. Each plot shows a nucleoporin-GFP mock- and WGA-treated. (b) mNup54-GFP constructs (top to bottom): tip, middle, boundary, folded. Mock treatment (dark blue, light gray in print); WGA treatment (light blue, gray in print). (c) mNup62-GFP constructs (top to bottom): tip, middle, boundary, folded. Mock treatment (maroon, light gray in print); WGA treatment (red, gray in print). All plots are mean ± SE.
Figure 6
Active transport and cargo binding have no effect on anisotropy. (a) Azide and deoxyglucose transport block monitored by mCherry-NLS. Accumulation of fluorescence in the nucleus is indicative of active transport. (b) Average ratio of anisotropy values for azide-treated/control yeast cells ± SD. (c) Standard deviation of average anisotropy ratio for azide-treated/control yeast cells. Smaller standard deviations indicate more similar values between the two conditions at all orientation points on the anisotropy curves. (d) Characteristic examples of Kap_β_-GFP unbinding in control cells (top three panels) and RanGTP-treated cells (bottom three panels). Nup62-mCherry is a marker for the NE and a control for fluorescence loss caused by photobleaching. Data from multiple cells was averaged and is shown in panel e. (e) Normalized average NE fluorescence ± SD for kap_β_-GFP and mNup62-mCherry in RanGTP-treated and control cells. (f) Average % NE fluorescence remaining ± SD 10 min after mock treatment or RanGTP. (g) Average ratio of anisotropy values for RanGTP-treated/control HeLa cells ± SD. (h) Standard deviation of average anisotropy ratio for RanGTP-treated/control HeLa cells.
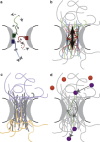
Figure 7
Speculative model for order of FG domains within the NPC. Different FG domains are shown as different colored ribbons. (a) Each FG domain has a unique order within the NPC depending on its properties and interactions. FG domains of the central nups increase in order from tip to base (gray arrows). (b) Dense packing of the central FG domains within NPC lumen results in their overall alignment. This alignment is characterized by overall orientational, but not positional, ordering. Individual FG domains can adopt multiple conformations but average orientation (black arrow) is maintained. (c) Peripheral FG domains have more freedom in the space they can explore. These domains are disordered on average. (d) Speculative model for Brownian ratchet cargo transport mechanism. Dense packing of the FG domains (gray ribbons) sterically prevents nonspecific molecules from entering (orange). Cargo bound to a karyopherin (purple) can recognize and bind FG repeats (1), allowing it to enter. The FG domain to which it is bound (green) changes conformation and moves across the NPC lumen (2). Cargo is released by RanGTP binding inside the nucleus (3).
Comment in
- Peptide ordering within nuclear pores in living cells.
Holz RW. Holz RW. Biophys J. 2013 Jan 8;104(1):6-7. doi: 10.1016/j.bpj.2012.11.3824. Epub 2013 Jan 8. Biophys J. 2013. PMID: 23332053 Free PMC article. No abstract available.
Similar articles
- The mechanism of nucleocytoplasmic transport through the nuclear pore complex.
Tetenbaum-Novatt J, Rout MP. Tetenbaum-Novatt J, et al. Cold Spring Harb Symp Quant Biol. 2010;75:567-84. doi: 10.1101/sqb.2010.75.033. Epub 2011 Mar 29. Cold Spring Harb Symp Quant Biol. 2010. PMID: 21447814 - Natively unfolded nucleoporins gate protein diffusion across the nuclear pore complex.
Patel SS, Belmont BJ, Sante JM, Rexach MF. Patel SS, et al. Cell. 2007 Apr 6;129(1):83-96. doi: 10.1016/j.cell.2007.01.044. Cell. 2007. PMID: 17418788 - A coarse-grained computational model of the nuclear pore complex predicts Phe-Gly nucleoporin dynamics.
Pulupa J, Rachh M, Tomasini MD, Mincer JS, Simon SM. Pulupa J, et al. J Gen Physiol. 2017 Oct 2;149(10):951-966. doi: 10.1085/jgp.201711769. Epub 2017 Sep 8. J Gen Physiol. 2017. PMID: 28887410 Free PMC article. - Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport.
Terry LJ, Wente SR. Terry LJ, et al. Eukaryot Cell. 2009 Dec;8(12):1814-27. doi: 10.1128/EC.00225-09. Epub 2009 Oct 2. Eukaryot Cell. 2009. PMID: 19801417 Free PMC article. Review. - Deciphering the intrinsically disordered characteristics of the FG-Nups through the lens of polymer physics.
Matsuda A, Mansour A, Mofrad MRK. Matsuda A, et al. Nucleus. 2024 Dec;15(1):2399247. doi: 10.1080/19491034.2024.2399247. Epub 2024 Sep 16. Nucleus. 2024. PMID: 39282864 Free PMC article. Review.
Cited by
- Improving the Hole Picture: Towards a Consensus on the Mechanism of Nuclear Transport.
Cowburn D, Rout M. Cowburn D, et al. ArXiv [Preprint]. 2023 Apr 6:arXiv:2304.03230v1. ArXiv. 2023. PMID: 37064528 Free PMC article. Updated. Preprint. - The molecular mechanism of nuclear transport revealed by atomic-scale measurements.
Hough LE, Dutta K, Sparks S, Temel DB, Kamal A, Tetenbaum-Novatt J, Rout MP, Cowburn D. Hough LE, et al. Elife. 2015 Sep 15;4:e10027. doi: 10.7554/eLife.10027. Elife. 2015. PMID: 26371551 Free PMC article. - In vivo analysis of protein crowding within the nuclear pore complex in interphase and mitosis.
Konishi HA, Asai S, Watanabe TM, Yoshimura SH. Konishi HA, et al. Sci Rep. 2017 Jul 18;7(1):5709. doi: 10.1038/s41598-017-05959-w. Sci Rep. 2017. PMID: 28720791 Free PMC article. - The nuclear pore complex core scaffold and permeability barrier: variations of a common theme.
Hayama R, Rout MP, Fernandez-Martinez J. Hayama R, et al. Curr Opin Cell Biol. 2017 Jun;46:110-118. doi: 10.1016/j.ceb.2017.05.003. Epub 2017 Jun 15. Curr Opin Cell Biol. 2017. PMID: 28624666 Free PMC article. Review. - Physics of the Nuclear Pore Complex: Theory, Modeling and Experiment.
Hoogenboom BW, Hough LE, Lemke EA, Lim RYH, Onck PR, Zilman A. Hoogenboom BW, et al. Phys Rep. 2021 Jul 25;921:1-53. doi: 10.1016/j.physrep.2021.03.003. Epub 2021 Mar 24. Phys Rep. 2021. PMID: 35892075 Free PMC article.
References
- Ryan K.J., Wente S.R. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 2000;12:361–371. - PubMed
- Moore M.S., Blobel G. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature. 1993;365:661–663. - PubMed
- Bischoff F.R., Ponstingl H. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 1991;354:80–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases