Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy - PubMed (original) (raw)
doi: 10.1016/j.neurobiolaging.2012.12.011. Epub 2013 Jan 16.
Daniel C Lee, Jerry B Hunt Jr, Josh M Morganti, Maj-Linda Selenica, Peter Moran, Patrick Reid, Milene Brownlow, Clement Guang-Yu Yang, Miloni Savalia, Carmelina Gemma, Paula C Bickford, Marcia N Gordon, David Morgan
Affiliations
- PMID: 23332170
- PMCID: PMC8970215
- DOI: 10.1016/j.neurobiolaging.2012.12.011
Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy
Kevin R Nash et al. Neurobiol Aging. 2013 Jun.
Abstract
Alzheimer's disease is characterized by amyloid plaques, neurofibrillary tangles, glial activation, and neurodegeneration. In mouse models, inflammatory activation of microglia accelerates tau pathology. The chemokine fractalkine serves as an endogenous neuronal modulator to quell microglial activation. Experiments with fractalkine receptor null mice suggest that fractalkine signaling diminishes tau pathology, but exacerbates amyloid pathology. Consistent with this outcome, we report here that soluble fractalkine overexpression using adeno-associated viral vectors significantly reduced tau pathology in the rTg4510 mouse model of tau deposition. Furthermore, this treatment reduced microglial activation and appeared to prevent neurodegeneration normally found in this model. However, in contrast to studies with fractalkine receptor null mice, parallel studies in an APP/PS1 model found no effect of increased fractalkine signaling on amyloid deposition. These data argue that agonism at fractalkine receptors might be an excellent target for therapeutic intervention in tauopathies, including those associated with amyloid deposition.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure statement
The authors have no conflicts of interest.
Figures
Fig. 1.
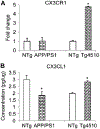
Fractalkine ligand (CX3CL1) and receptor(CX3CR1) levels in transgenic mice. (A) Western blot with anti-CX3CR1 antibody using hippocampal cell lysates of APP/PS1, Tg4510, or nontransgenic (NTg) littermate controls and normalizing to GAPDH staining; (B) Enzyme-linked immunosorbent assay for CX3CL1 of hippocampal cell lysates of APP/PS1, Tg4510, or nontransgenic littermate controls.
Fig. 2.
Expression of FKN constructs. (A) Western blot with anti-HA tag antibody using cell lysates of plasmid transfected HEK293 cells. (B) Anti-HA Western blot of the media from transfected HEK293 cells, only sFKN is observed in the media. (C and D) Anti-HA immunohistochemistry of hippocampus of GFP-injected and sFKN-injected mice. Scale = 200 μm. Abbreviations: DG, dentate gyrus; FKN, fractalkine; GFP, green fluorescent protein (control); HA, hemagglutinin; n, native (membrane associated) FKN; sFKN, secreted FKN.
Fig. 3.
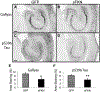
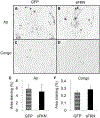
Tau pathology is reduced after sFKN transduction. Gallyas staining of rAAV-GFP (A) and rAAV-sFKN (B) -injected mice hippocampi. Staining with anti-phosphotau 396 antibody in rAAV-GFP (C) and rAAV-sFKN (D) -injected mice. Percentage area positive staining for Gallyas (E) and anti-phopsphotau 396 (F). ** P < 0.01; * P < 0.05. Scale = 200 μm. Abbreviations: FKN, fractalkine; GFP, green fluorescent protein; rAAV, recombinant adeno-associated virus serotype; sFKN, secreted FKN.
Fig. 4.
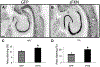
Anti-NeuN immunohistochemistry is increased after rAAV-sFKN treatment. Staining with anti-NeuN antibody in rAAV-GFP (A) and rAAV-sFKN (B) -injected mice. Percentage area positive staining for anti-NeuN in the CA1–CA3 of HPC (C) or DG subfield (D). * P < 0.05. Scale = 200 μm. Abbreviations: DG, dentate gyrus; FKN, fractalkine; GFP, green fluorescent protein; HPC, hippocampus; rAAV, recombinant adeno-associated virus serotype; sFKN, secreted FKN.
Fig. 5.
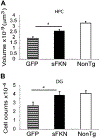
Stereological analysis demonstrates a reduction in neuron loss in the AAV-sFKN treated group compared with the AAV-GFP treated group. HPC volume (μm3) (A) and granular cell counts of the DG (B). * P < 0.02. Abbreviations: AAV, adeno-associated virus serotype; DG, dentate gyrus; FKN, fractalkine; GFP, green fluorescent protein; HPC, hippocampus; NonTg, nontransgenic; sFKN, secreted FKN.
Fig. 6.
Western blot analysis shows reduced phospho-tau levels. (A) Intensity of staining is represented as fold change over the control GFP treated group after standardizing to GAPDH staining. Total tau (H150 and HT7 antibody), anti-pS199/S202, pS262, pS396, and pS356 tau; anti-GSK3 α;*216/β279, and anti-PHF tau antibodies AT8, AT180, and AT270 are shown. (B) Representative Western blot images of panel A. (C) Fold change in intensity for the formic acid tau fraction. * P < 0.01. Abbreviations: FKN, fractalkine; GAPDH, glyc-eraldehyde-3-phosphate dehydrogenase; GFP, green fluorescent protein; PHF, pair helical filament; sFKN, secreted FKN.
Fig. 7.
Anti-CD45 immunohistochemistry is reduced after rAAV-sFKN treatment. Anti-CD45 staining of rAAV-GFP (A) and rAAV-sFKN (B) -injected mice hippocampi (scale = 200 μm). Morphology of CD45-stained cells in an rAAV-GFP injected mouse (scale = 50 μm) (C). Percentage area positive staining for anti-CD45 (D). * P < 0.02. Abbreviations: AAV, adeno-associated virus serotype; FKN, fractalkine; GFP, green fluorescent protein; rAAV, recombinant AAV; sFKN, secreted FKN.
Fig. 8.
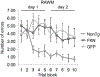
RAWM test demonstrates no phenotype rescue with AAV-sFKN treatment in the hippocampus compared with nonTg and AAV-GFP treated Tg4510 mice. Number of errors is shown as an average of every 3 trials as 1 block. Abbreviations: AAV, adeno-associated virus serotype; FKN, fractalkine; GFP, green fluorescent protein; nonTG, nontransgenic; RAWM, radial arm water maze; sFKN, secreted FKN.
Fig. 9.
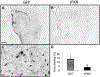
Amyloid levels in APP/PS1 mice treated with GFP or sFKN virus are unaltered. Anti-Aβ staining of rAAV-GFP (A) and rAAV-sFKN (B) -injected mice hippocampi. Congo red staining of rAAV-GFP (C) and rAAV-sFKN (D) -injected mice hippocampi. Percentage area positive staining for anti-Aβ (E) and Congo red staining (F). Scale = 200 μm. Abbreviations: Aβ, amyloid beta; AAV, adeno-associated virus serotype; FKN, fractalkine; GFP, green fluorescent protein; rAAV, recombinant AAV; sFKN, secreted FKN.
Similar articles
- Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Genetically enhancing the expression of chemokine domain of CX3CL1 fails to prevent tau pathology in mouse models of tauopathy.
Bemiller SM, Maphis NM, Formica SV, Wilson GN, Miller CM, Xu G, Kokiko-Cochran ON, Kim KW, Jung S, Cannon JL, Crish SD, Cardona AE, Lamb BT, Bhaskar K. Bemiller SM, et al. J Neuroinflammation. 2018 Sep 25;15(1):278. doi: 10.1186/s12974-018-1310-6. J Neuroinflammation. 2018. PMID: 30253780 Free PMC article. - CCL2 Overexpression in the Brain Promotes Glial Activation and Accelerates Tau Pathology in a Mouse Model of Tauopathy.
Joly-Amado A, Hunter J, Quadri Z, Zamudio F, Rocha-Rangel PV, Chan D, Kesarwani A, Nash K, Lee DC, Morgan D, Gordon MN, Selenica MB. Joly-Amado A, et al. Front Immunol. 2020 May 20;11:997. doi: 10.3389/fimmu.2020.00997. eCollection 2020. Front Immunol. 2020. PMID: 32508844 Free PMC article. - CNS-Wide over Expression of Fractalkine Improves Cognitive Functioning in a Tauopathy Model.
Finneran DJ, Morgan D, Gordon MN, Nash KR. Finneran DJ, et al. J Neuroimmune Pharmacol. 2019 Jun;14(2):312-325. doi: 10.1007/s11481-018-9822-5. Epub 2018 Nov 29. J Neuroimmune Pharmacol. 2019. PMID: 30499006 Free PMC article. - The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies.
Goedert M. Goedert M. Alzheimers Dement. 2016 Oct;12(10):1040-1050. doi: 10.1016/j.jalz.2016.09.001. Epub 2016 Sep 26. Alzheimers Dement. 2016. PMID: 27686274 Review.
Cited by
- Bridging the Gap Between Fluid Biomarkers for Alzheimer's Disease, Model Systems, and Patients.
Bjorkli C, Sandvig A, Sandvig I. Bjorkli C, et al. Front Aging Neurosci. 2020 Sep 2;12:272. doi: 10.3389/fnagi.2020.00272. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32982716 Free PMC article. Review. - Neuroinflammation in Alzheimer's disease: chemokines produced by astrocytes and chemokine receptors.
Liu C, Cui G, Zhu M, Kang X, Guo H. Liu C, et al. Int J Clin Exp Pathol. 2014 Dec 1;7(12):8342-55. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25674199 Free PMC article. Review. - Therapeutic Targets in Innate Immunity to Tackle Alzheimer's Disease.
Serradas ML, Ding Y, Martorell PV, Kulińska I, Castro-Gomez S. Serradas ML, et al. Cells. 2024 Aug 26;13(17):1426. doi: 10.3390/cells13171426. Cells. 2024. PMID: 39272998 Free PMC article. Review. - Therapeutic Delivery of Soluble Fractalkine Ameliorates Vascular Dysfunction in the Diabetic Retina.
Rodriguez D, Church KA, Smith CT, Vanegas D, Cardona SM, Muzzio IA, Nash KR, Cardona AE. Rodriguez D, et al. Int J Mol Sci. 2024 Jan 31;25(3):1727. doi: 10.3390/ijms25031727. Int J Mol Sci. 2024. PMID: 38339005 Free PMC article. - Two forms of CX3CL1 display differential activity and rescue cognitive deficits in CX3CL1 knockout mice.
Winter AN, Subbarayan MS, Grimmig B, Weesner JA, Moss L, Peters M, Weeber E, Nash K, Bickford PC. Winter AN, et al. J Neuroinflammation. 2020 May 14;17(1):157. doi: 10.1186/s12974-020-01828-y. J Neuroinflammation. 2020. PMID: 32410624 Free PMC article.
References
- Aisen PS, Davis KL, Berg JD, Schafer K, Campbell K, Thomas RG, Weiner MF, Farlow MR, Sano M, Grundman M, Thal LJ, 2000. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology 54, 588–593. - PubMed
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ, 2003. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289, 2819–2826. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. - PMC - PubMed
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D, 2006. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat. Protoc 1,1671–1679. - PubMed
- Arendash GW, King DL, Gordon MN, Morgan D, Hatcher JM, Hope CE, Diamond DM, 2001. Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 891, 42–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous