Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands - PubMed (original) (raw)
. 2013 Jan 16;4(1):96-109.
doi: 10.1021/cn3000668. Epub 2012 Jul 17.
Affiliations
- PMID: 23336049
- PMCID: PMC3547484
- DOI: 10.1021/cn3000668
Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands
Jose I Juncosa Jr et al. ACS Chem Neurosci. 2013.
Abstract
Based on the structure of the superpotent 5-HT(2A) agonist 2-(4-bromo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine, which consists of a ring-substituted phenethylamine skeleton modified with an N-benzyl group, we designed and synthesized a small library of constrained analogues to identify the optimal arrangement of the pharmacophoric elements of the ligand. Structures consisted of diversely substituted tetrahydroisoquinolines, piperidines, and one benzazepine. Based on the structure of (S,S)-9b, which showed the highest affinity of the series, we propose an optimal binding conformation. (S,S)-9b also displayed 124-fold selectivity for the 5-HT(2A) over the 5-HT(2C) receptor, making it the most selective 5-HT(2A) receptor agonist ligand currently known.
Figures
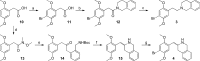
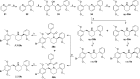
Scheme 1
Reagents and conditions: (a) Br2, CH3COOH, rt, 3 h, 68%; (b) i. SOCl2, C6H6, reflux, 1 h; ii. 1,2,3,4-tetrahydroisoquinoline, NEt3, CH2Cl2, reflux, 2 h, 75% (cis/trans = 0.72); (c) BH3.THF, THF, 0 °C, 1 h, then rt, 4 h; then 2 M HCl in EtOH, reflux, 15 h, 52%; (d) i. (COCl)2, CHCl3, rt, 1 h; ii. N,_O_-dimethylhydroxylamine hydrochloride, pyridine, rt, 1 h, 87%; (e) _tert_-butyl 2-methylbenzylcarbamate, _s-_BuLi, THF, −40 °C, 15 min, then 13, THF, −65 °C to rt, 2 h, 38%; (f) i. CF3COOH, CH2Cl2, rt, 30 min; ii. NaBH4, EtOH, rt, 1 h, 75%; (g) Br2, 1:1 CH3COOH/1,4-dioxane, 0 °C to rt, 48 h, 51%.
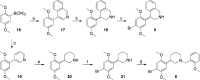
Scheme 2
Reagents and conditions: (a) 4-bromoisoquinoline, Pd(PPh3)4, Na2CO3, EtOH, C6H6, H2O, reflux, 4 h, 72%; (b) NaBH3CN, HCl, MeOH, rt, 99%; (c) Br2, CH3COOH, rt, 15 h, 58%; (d) 10% Pd/C, PPh3, Na2CO3, DME, H2O, 80 °C, 15 h, 78%; (e) H2 (50–70 psi), PtO2, CH3COOH, rt, 6 h, 88%; (f) Br2, 1:1 CH3COOH/1,4-dioxane, 0 °C to rt, 18 h, 62%; (g) _o_-anisaldehyde, MeOH, 3 Å MS, NaBH3CN, rt, 6 h, 66% (99% BRSM).
Scheme 3
Reagents and conditions: (a) _s_-BuLi, THF, −45 °C, 45 min, then 2,5-dimethoxy-β-nitrostyrene, THF, −45 °C to rt, 30 min, 60%; (b) 2 N HCl (aq), THF, reflux, 15 h, 74%; (c) i. Zn, CH3COOH, rt, 48 h; ii. MeOH, reflux, 15 h, 58%; (d) BH3.THF, THF, reflux, 24 h; then 2 M HCl in EtOH, reflux, 12 h, 96%; (e) Br2, CH3COOH, rt, 15 h, 71%;
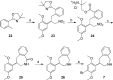
Scheme 4
Reagents and conditions: (a) 16, Pd(PPh3)4, Na2CO3, PhCH3, H2O, 65 °C, 24 h, 48%; (b) _o_-anisylmagnesium bromide, THF, Ni(acac)2, dppe, rt, 24 h, 77%; (c) Na, EtOH, reflux, 3 h, 38% (30a), 38% (30b); (d) Br2, 1:1 CH3COOH/1,4-dioxane, 0 °C to rt, 18 h, 66% (8a), 87% (8b).
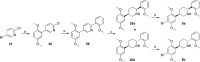
Scheme 5
Reagents and conditions: (a) _o_-anisylmagnesium bromide, THF, Ni(acac)2, dppe, rt, 18 h, 77%; (b) _n_-BuLi:LiDMAE, PhCH3, −40 °C, 1 h, then 2,5-dimethoxybenzaldehyde, THF, −78 °C to rt, 30 min, 46%; (c) H2 (50 psi), 10% Pd/C, HCl (aq), MeOH, rt, 10 days, 72%; (d) H2 (60 psi), PtO2, CH3COOH, rt, 3 h, 85%; (e) Na, EtOH, reflux, 3 h, 38% (35a), 49% (35b); (f) Br2, 1:1 CH3COOH/1,4-dioxane, 0 °C to rt, 15 h (9a) or 63 h (9b), 61% (9a), 64% (9b); (g) _O_-methylmandeloyl chloride, NaOH, H2O, CH2Cl2, rt, 1.5 h, 28% (36a), 28% (36b); (h) LiEt3BH, THF, rt, 3 days (R,_R_-9b) or 7 days (S,_S_-9b), 66% (R,_R_-9b), 63% (S,_S_-9b).
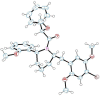
Figure 1
ORTEP view of compound 36a, obtained by X-ray crystallography.
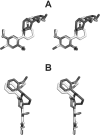
Figure 2
Stereoviews (crossed-eye) of the structure of (S,S)-9b (dark gray) in the proposed bioactive conformation after docking into a homology model of the human 5-HT2A serotonin receptor, superimposed on the structure of (S)-6 (light gray). A is the front view, and B is the top view. The extracellular side of the receptor is toward the top of panel A.
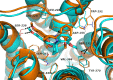
Figure 3
Compounds (R,R) and (S,S)-9b (orange and blue, respectively) in the simulated 5-HT2A receptor binding site.
Figure 4
Comparison between the simulated binding poses of compounds 2 (green) and (S,S)-9b (blue) in the binding site of the 5-HT2A receptor.
Similar articles
- 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists.
McLean TH, Parrish JC, Braden MR, Marona-Lewicka D, Gallardo-Godoy A, Nichols DE. McLean TH, et al. J Med Chem. 2006 Sep 21;49(19):5794-803. doi: 10.1021/jm060656o. J Med Chem. 2006. PMID: 16970404 - Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers.
Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Ettrup A, et al. Eur J Nucl Med Mol Imaging. 2011 Apr;38(4):681-93. doi: 10.1007/s00259-010-1686-8. Epub 2010 Dec 21. Eur J Nucl Med Mol Imaging. 2011. PMID: 21174090 - Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors.
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Johnson RA, Janowsky A. Eshleman AJ, et al. Biochem Pharmacol. 2018 Dec;158:27-34. doi: 10.1016/j.bcp.2018.09.024. Epub 2018 Sep 25. Biochem Pharmacol. 2018. PMID: 30261175 Free PMC article. - Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens.
Halberstadt AL. Halberstadt AL. Curr Top Behav Neurosci. 2017;32:283-311. doi: 10.1007/7854_2016_64. Curr Top Behav Neurosci. 2017. PMID: 28097528 Review. - 25CN-NBOH: A Selective Agonist for in vitro and in vivo Investigations of the Serotonin 2A Receptor.
Märcher Rørsted E, Jensen AA, Kristensen JL. Märcher Rørsted E, et al. ChemMedChem. 2021 Nov 5;16(21):3263-3270. doi: 10.1002/cmdc.202100395. Epub 2021 Aug 21. ChemMedChem. 2021. PMID: 34288515 Review.
Cited by
- Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents.
Gatch MB, Dolan SB, Forster MJ. Gatch MB, et al. Behav Pharmacol. 2017 Aug;28(5):375-385. doi: 10.1097/FBP.0000000000000309. Behav Pharmacol. 2017. PMID: 28537942 Free PMC article. - Celebrating serotonin.
Andrews AM. Andrews AM. ACS Chem Neurosci. 2012 Sep 19;3(9):644-5. doi: 10.1021/cn300137q. ACS Chem Neurosci. 2012. PMID: 23019490 Free PMC article. No abstract available. - Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species.
Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Halberstadt AL, et al. Neuropharmacology. 2020 May 1;167:107933. doi: 10.1016/j.neuropharm.2019.107933. Epub 2020 Jan 7. Neuropharmacology. 2020. PMID: 31917152 Free PMC article. - Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists.
Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. Hansen M, et al. ACS Chem Neurosci. 2014 Mar 19;5(3):243-9. doi: 10.1021/cn400216u. Epub 2014 Jan 15. ACS Chem Neurosci. 2014. PMID: 24397362 Free PMC article. - Recent advances in the neuropsychopharmacology of serotonergic hallucinogens.
Halberstadt AL. Halberstadt AL. Behav Brain Res. 2015 Jan 15;277:99-120. doi: 10.1016/j.bbr.2014.07.016. Epub 2014 Jul 15. Behav Brain Res. 2015. PMID: 25036425 Free PMC article. Review.
References
- Fujii Y.; Saitoh Y.; Tanaka H.; Toyooka H. (2000) Ramosetron for preventing postoperative nausea and vomiting in women undergoing gynecological surgery. Anesth. Analg. 90, 472–475. - PubMed
- Nichols D. E.; Nichols C. D. (2008) Serotonin receptors. Chem. Rev. 108, 1614–1641. - PubMed
- Siegel G. J., and Agranoff B. W. (1999) Basic neurochemistry: molecular, cellular, and medical aspects, 6th ed., p xxi, 1183, Lippincott-Raven Publishers, Philadelphia.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources