Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance - PubMed (original) (raw)
Review
Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance
Rocío García-Becerra et al. Int J Mol Sci. 2012.
Abstract
Breast cancer is the most frequent malignancy diagnosed in women. Approximately 70% of breast tumors express the estrogen receptor (ER). Tamoxifen and aromatase inhibitors (AIs) are the most common and effective therapies for patients with ERα-positive breast cancer. Alone or combined with chemotherapy, tamoxifen significantly reduces disease progression and is associated with more favorable impact on survival in patients. Unfortunately, endocrine resistance occurs, either de novo or acquired during the course of the treatment. The mechanisms that contribute to hormonal resistance include loss or modification in the ERα expression, regulation of signal transduction pathways, altered expression of specific microRNAs, balance of co-regulatory proteins, and genetic polymorphisms involved in tamoxifen metabolic activity. Because of the clinical consequences of endocrine resistance, new treatment strategies are arising to make the cells sensitive to tamoxifen. Here, we will review the current knowledge on mechanisms of endocrine resistance in breast cancer cells. In addition, we will discuss novel therapeutic strategies to overcome such resistance. Undoubtedly, circumventing endocrine resistance should help to improve therapy for the benefit of breast cancer patients.
Figures
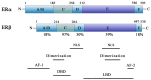
Figure 1
Schematic representation of functional domains of human ERα and ERβ. The A/B domain at the _N_-terminal contains AF-1 site. The C domain includes the DNA-binding domain (DBD) and a dimerization site. The D domain contains a nuclear localization signal. The E/F domain is located at the _C_-terminal and comprises the ligand binding, as well as the AF-2 domain, a second nuclear localization signal, and another dimerization site.
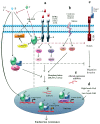
Figure 2
Pathways involved in endocrine resistance. (a) While tamoxifen (T), aromatase inhibitors (AIs), or fulvestrant (F) inhibit estrogen (E) signalization, GFR pathways promote ER phosphorylation, transcription factors (TFs), and their coactivators (CoA) in a ligand-independent manner. E-ER complex outside the nucleus can interact with GFRs, Src, CoA and matrix metalloproteinases that release heparin-binding-EGF; (b) Stress may trigger signalization leading to ER and its coregulators phosphorylation; (c) Notch regulates the migration and invasion of breast cancer cells. E inhibits this pathway while T activates it; (d) High levels of CoA, low levels of corepressors (CoR) and altered expression of miRs (e) have been implicated in endocrine resistance development.
Similar articles
- Identification of miRNAs as biomarkers for acquired endocrine resistance in breast cancer.
Muluhngwi P, Klinge CM. Muluhngwi P, et al. Mol Cell Endocrinol. 2017 Nov 15;456:76-86. doi: 10.1016/j.mce.2017.02.004. Epub 2017 Feb 3. Mol Cell Endocrinol. 2017. PMID: 28163101 Review. - Loss of Estrogen-Regulated MIR135A1 at 3p21.1 Promotes Tamoxifen Resistance in Breast Cancer.
Zhang W, Wu M, Chong QY, Zhang M, Zhang X, Hu L, Zhong Y, Qian P, Kong X, Tan S, Li G, Ding K, Lobie PE, Zhu T. Zhang W, et al. Cancer Res. 2018 Sep 1;78(17):4915-4928. doi: 10.1158/0008-5472.CAN-18-0069. Epub 2018 Jun 26. Cancer Res. 2018. PMID: 29945962 - Understanding response and resistance to oestrogen deprivation in ER-positive breast cancer.
Patani N, Martin LA. Patani N, et al. Mol Cell Endocrinol. 2014 Jan 25;382(1):683-694. doi: 10.1016/j.mce.2013.09.038. Epub 2013 Oct 9. Mol Cell Endocrinol. 2014. PMID: 24121024 Review. - The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: A systematic review and meta-analysis of randomized and non-randomized trials.
Najim O, Seghers S, Sergoynne L, Van Gaver H, Papadimitriou K, Wouters K, Trinh XB, Huizing MT, Tjalma W. Najim O, et al. Biochim Biophys Acta Rev Cancer. 2019 Dec;1872(2):188315. doi: 10.1016/j.bbcan.2019.188315. Epub 2019 Oct 21. Biochim Biophys Acta Rev Cancer. 2019. PMID: 31647985 Review. - Long Non-Coding RNA H19 Acts as an Estrogen Receptor Modulator that is Required for Endocrine Therapy Resistance in ER+ Breast Cancer Cells.
Basak P, Chatterjee S, Bhat V, Su A, Jin H, Lee-Wing V, Liu Q, Hu P, Murphy LC, Raouf A. Basak P, et al. Cell Physiol Biochem. 2018;51(4):1518-1532. doi: 10.1159/000495643. Epub 2018 Nov 29. Cell Physiol Biochem. 2018. PMID: 30497079
Cited by
- Estrogen Receptor Expression Is Associated with DNA Repair Capacity in Breast Cancer.
Matta J, Morales L, Ortiz C, Adams D, Vargas W, Casbas P, Dutil J, Echenique M, Suárez E. Matta J, et al. PLoS One. 2016 Mar 31;11(3):e0152422. doi: 10.1371/journal.pone.0152422. eCollection 2016. PLoS One. 2016. PMID: 27032101 Free PMC article. - Clinical Outcomes of Patients Treated with Ribociclib in Combination with Aromatase Inhibitors or Fulvestrant for HR-Positive, HER2-Negative Metastatic Breast Cancer, Real-World Data from a Low-Resourced Country.
Abdel-Razeq H, Sharaf B, Khater S, Baidoun HJ, Bani Hani H, Taqash A, El Khatib O, Edaily S, Abunasser M, Tamimi F, Al-Masri YN, Al-Batsh TMW, Zayed A, Ghatasheh T, Radaideh T. Abdel-Razeq H, et al. Immunotargets Ther. 2024 Sep 29;13:501-512. doi: 10.2147/ITT.S479153. eCollection 2024. Immunotargets Ther. 2024. PMID: 39364228 Free PMC article. - Automated Quantification of Extranuclear ERα using Phosphor-integrated Dots for Predicting Endocrine Therapy Resistance in HR+/HER2- Breast Cancer.
Guo Z, Tada H, Kitamura N, Hamada Y, Miyashita M, Harada-Shoji N, Sato A, Hamanaka Y, Tsuboi K, Harada N, Takano-Kasuya M, Okada H, Nakano Y, Ohuchi N, Hayashi SI, Ishida T, Gonda K. Guo Z, et al. Cancers (Basel). 2019 Apr 12;11(4):526. doi: 10.3390/cancers11040526. Cancers (Basel). 2019. PMID: 31013810 Free PMC article. - Review of hormone-based treatments in postmenopausal patients with advanced breast cancer focusing on aromatase inhibitors and fulvestrant.
Kümler I, Knoop AS, Jessing CA, Ejlertsen B, Nielsen DL. Kümler I, et al. ESMO Open. 2016 Aug 16;1(4):e000062. doi: 10.1136/esmoopen-2016-000062. eCollection 2016. ESMO Open. 2016. PMID: 27843622 Free PMC article. Review. - Clinical relevance of Cyr61 expression in patients with hormone-dependent breast cancer.
Mayer S, Erbes T, Timme-Bronsert S, Jaeger M, Rücker G, Kuf F, Stickeler E, Gitsch G, Hirschfeld M. Mayer S, et al. Oncol Lett. 2017 Aug;14(2):2334-2340. doi: 10.3892/ol.2017.6406. Epub 2017 Jun 16. Oncol Lett. 2017. PMID: 28789451 Free PMC article.
References
- Clark G.M., Osborne C.K., McGuire W.L. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J. Clin. Oncol. 1984;2:1102–1109. - PubMed
- Miller W.R., Bartlett J.M., Canney P., Verrill M. Hormonal therapy for postmenopausal breast cancer: the science of sequencing. Breast Cancer Res. Treat. 2007;103:149–160. - PubMed
- Osborne C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339:1609–1618. - PubMed
- Ring A., Dowsett M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer. 2004;11:643–658. - PubMed
- Gradishar W.J. Tamoxifen—What next? Oncologist. 2004;9:378–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical