Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis - PubMed (original) (raw)
Review
Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis
J Charles Jennette et al. Annu Rev Pathol. 2013.
Abstract
Clinical, in vitro, and experimental animal observations indicate that antineutrophil cytoplasmic autoantibodies (ANCA) are pathogenic. The genesis of the ANCA autoimmune response is a multifactorial process that includes genetic predisposition, environmental adjuvant factors, an initiating antigen, and failure of T cell regulation. ANCA activate primed neutrophils (and monocytes) by binding to certain antigens expressed on the surface of neutrophils in specific inflammatory microenvironments. ANCA-activated neutrophils activate the alternative complement pathway, establishing an inflammatory amplification loop. The acute injury elicits an innate inflammatory response that recruits monocytes and T lymphocytes, which replace the neutrophils that have undergone karyorrhexis during acute inflammation. Extravascular granulomatous inflammation may be initiated by ANCA-induced activation of extravascular neutrophils, causing tissue necrosis and fibrin formation, which would elicit an influx of monocytes that transform into macrophages and multinucleated giant cells. Over time, the neutrophil-rich acute necrotizing lesions cause the accumulation of more lymphocytes, monocytes, and macrophages and produce typical granulomatous inflammation.
Figures
Figure 1
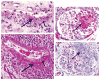
Segmental acute necrotizing ANCA-associated vasculitis lesions with (a_–_c) fibrinoid necrosis (hematoxylin and eosin stain) (large arrow) and (a,c) leukocytoclasia (small arrow). (a,b) The inflammatory infiltrate includes a mixture of neutrophils and mononuclear leukocytes. (c,d ) A Masson trichrome stain is useful in distinguishing between (b) acute segmental fibrinoid necrosis and (d) chronic segmental sclerosis.
Figure 2
Acute pulmonary lesions in (a,b) microscopic polyangiitis and (c,d) granulomatosis with polyangiitis (GPA). (a) Alveolar capillaritis with extensive neutrophilic infiltration and hemorrhage [hematoxylin and eosin (H&E) stain]. (b) Extensive disruption of the silver-positive alveolar capillary basement membranes in the center of the photomicrograph (Jones silver stain). (c) An acute GPA granulomatous lesion with a central zone of intense neutrophilic infiltration (microabscess) with adjacent multinucleated giant cells (H&E stain). (d) A more chronic GPA granulomatous lesion with necrotic debris and adjacent palisading macrophages (H&E stain).
Figure 3
Glomerulonephritis and vasculitis induced in a B6 mouse 6 days after intravenous injection of mouse anti-MPO (myeloperoxidase) immunoglobulin G. (a) Segmental necrotizing glomerulonephritis with fibrinoid necrosis [hematoxylin and eosin (H&E stain)]. (b) Immunohistochemical staining of neutrophils showing segmental accumulation at sites of necrosis. (c) Leukocytoclastic angiitis in the skin (H&E stain). (d) Hemorrhagic pulmonary alveolar capillaritis (Masson trichrome stain).
Figure 4
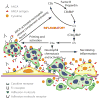
The putative pathogenic event in acute ANCA-associated vasculitis. (Left to right) Neutrophils that have been primed (e.g., with cytokines) release ANCA antigens at the cell surface and into the adjacent microenvironment, where they bind ANCA. Activated neutrophils release factors that activate the alternative complement pathway, which in turn recruits and primes more neutrophils for activation by ANCA and further activation of complement, resulting in an amplification loop. ANCA-activated neutrophils adhere to and penetrate vessel walls and release destructive mediators that cause vascular necrosis.
Figure 5
Multiple events contributing to the pathogenesis of ANCA-associated vasculitis, including (a) genesis of the autoimmune response by an inciting antigen, (b) loss of tolerance that allows the autoimmune response to persist, (c) abnormally increased expression of ANCA target antigens in neutrophils, and (d) cytokine-induced increased release to ANCA antigens at the surface of neutrophils and into the inflammatory microenvironment. The autoimmune response is initiated by a peptide antigen (Ag1) that is complementary to the autoantigen (Ag2). The responding B cells (B1) produce antibodies (A1) directed against the complementary peptide. The A1 antibodies stimulate an anti-idiotypic response (B2, A2) that cross-reacts with the autoantigen (Ag2). Perpetuation of the pathogenic anti-Ag2 response requires loss of tolerance due to dysfunction of regulatory T cells (Tregs).
Figure 6
A putative sequence of events in the pathogenesis of ANCA-associated vasculitis. (Top to bottom) Loss of tolerance allows for production of pathogenic levels of ANCA. ANCA activate primed neutrophils by binding to ANCA antigens at the surface of neutrophils and in the microenvironment of the inflammation. ANCA-activated neutrophils mediate acute necrotizing injury with fibrinoid necrosis and leukocytoclasia (compare with Figure 1_a_). The acute injury elicits an innate inflammatory response that recruits monocytes and T lymphocytes, which replace the neutrophils and lead to either resolution of the injury or development of localized fibrosis/sclerosis.
Figure 7
Putative events in the pathogenesis of extravascular granulomatosis. (Upper left) Extravascular neutrophils are activated to produce (upper middle) intense localized acute inflammation, which causes (upper right) tissue necrosis and fibrin formation. The acute injury elicits a mononuclear leukocyte response, including (bottom) the influx of monocytes that transform into macrophages and multinucleated giant cells.
Similar articles
- Pathogenesis of ANCA-Associated Pulmonary Vasculitis.
Alba MA, Jennette JC, Falk RJ. Alba MA, et al. Semin Respir Crit Care Med. 2018 Aug;39(4):413-424. doi: 10.1055/s-0038-1673386. Epub 2018 Nov 7. Semin Respir Crit Care Med. 2018. PMID: 30404109 Free PMC article. Review. - Pathogenesis of ANCA-associated vasculitis: An update.
Jarrot PA, Kaplanski G. Jarrot PA, et al. Autoimmun Rev. 2016 Jul;15(7):704-13. doi: 10.1016/j.autrev.2016.03.007. Epub 2016 Mar 9. Autoimmun Rev. 2016. PMID: 26970490 Review. - B cell-mediated pathogenesis of ANCA-mediated vasculitis.
Jennette JC, Falk RJ. Jennette JC, et al. Semin Immunopathol. 2014 May;36(3):327-38. doi: 10.1007/s00281-014-0431-y. Epub 2014 Apr 29. Semin Immunopathol. 2014. PMID: 24777746 Free PMC article. Review. - The role of neutrophils in causing antineutrophil cytoplasmic autoantibody-associated vasculitis.
Schreiber A, Choi M. Schreiber A, et al. Curr Opin Hematol. 2015 Jan;22(1):60-6. doi: 10.1097/MOH.0000000000000098. Curr Opin Hematol. 2015. PMID: 25394311 Review. - Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease.
Jennette JC, Falk RJ. Jennette JC, et al. Nat Rev Rheumatol. 2014 Aug;10(8):463-73. doi: 10.1038/nrrheum.2014.103. Epub 2014 Jul 8. Nat Rev Rheumatol. 2014. PMID: 25003769 Review.
Cited by
- Anti-Pentraxin Antibodies in Autoimmune Diseases: Bystanders or Pathophysiological Actors?
Brilland B, Vinatier E, Subra JF, Jeannin P, Augusto JF, Delneste Y. Brilland B, et al. Front Immunol. 2021 Feb 16;11:626343. doi: 10.3389/fimmu.2020.626343. eCollection 2020. Front Immunol. 2021. PMID: 33664737 Free PMC article. Review. - Retinal drusen in glomerulonephritis with or without immune deposits suggest systemic complement activation in disease pathogenesis.
Harraka P, Mack H, Colville D, Barit D, Langsford D, Pianta T, Ierino F, Savige J. Harraka P, et al. Sci Rep. 2022 May 17;12(1):8234. doi: 10.1038/s41598-022-12111-w. Sci Rep. 2022. PMID: 35581312 Free PMC article. - Tofacitinib-Induced Antineutrophil Cytoplasmic Antibodies (ANCA)-Associated Vasculitis With Crescentic Glomerulonephritis.
Asemota U, Greenberg S, Gulati A, Kumar K, Janga K. Asemota U, et al. Cureus. 2021 Oct 11;13(10):e18663. doi: 10.7759/cureus.18663. eCollection 2021 Oct. Cureus. 2021. PMID: 34790443 Free PMC article. - Genetic Susceptibility to ANCA-Associated Vasculitis: State of the Art.
Bonatti F, Reina M, Neri TM, Martorana D. Bonatti F, et al. Front Immunol. 2014 Nov 17;5:577. doi: 10.3389/fimmu.2014.00577. eCollection 2014. Front Immunol. 2014. PMID: 25452756 Free PMC article. Review.
References
- Falk RJ, Jennette JC. ANCA disease: Where is this field going? J Am Soc Nephrol. 2010;21:745–52. - PubMed
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–57. Reports, for the first time, ANCA specificity for MPO, and recognizes multiple ANCA antigen specificities. - PubMed
- Jennette JC, Hoidal JH, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources