Primary cilia and aberrant cell signaling in epithelial ovarian cancer - PubMed (original) (raw)
Primary cilia and aberrant cell signaling in epithelial ovarian cancer
Dorte L Egeberg et al. Cilia. 2012.
Abstract
Background: Ovarian cancer is the fourth leading cause of cancer-related deaths among women in Denmark, largely due to the advanced stage at diagnosis in most patients. Approximately 90% of ovarian cancers originate from the single-layered ovarian surface epithelium (OSE). Defects in the primary cilium, a solitary sensory organelle in most cells types including OSE, were recently implicated in tumorigenesis, mainly due to deregulation of ciliary signaling pathways such as Hedgehog (Hh) signaling. However, a possible link between primary cilia and epithelial ovarian cancer has not previously been investigated.
Methods: The presence of primary cilia was analyzed in sections of fixed human ovarian tissue as well as in cultures of normal human ovarian surface epithelium (OSE) cells and two human OSE-derived cancer cell lines. We also used immunofluorescence microscopy, western blotting, RT-PCR and siRNA to investigate ciliary signaling pathways in these cells.
Results: We show that ovarian cancer cells display significantly reduced numbers of primary cilia. The reduction in ciliation frequency in these cells was not due to a failure to enter growth arrest, and correlated with persistent centrosomal localization of aurora A kinase (AURA). Further, we demonstrate that ovarian cancer cells have deregulated Hh signaling and platelet-derived growth factor receptor alpha (PDGFRα) expression and that promotion of ciliary formation/stability by AURA siRNA depletion decreases Hh signaling in ovarian cancer cells. Lastly, we show that the tumor suppressor protein and negative regulator of AURA, checkpoint with forkhead-associated and ring finger domains (CHFR), localizes to the centrosome/primary cilium axis.
Conclusions: Our results suggest that primary cilia play a role in maintaining OSE homeostasis and that the low frequency of primary cilia in cancer OSE cells may result in part from over-expression of AURA, leading to aberrant Hh signaling and ovarian tumorigenesis.
Figures
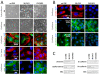
Figure 1
Characterization of ovarian surface epithelium. A) Section through a normal adult ovarian cortex stained with H & E, showing OSE cells at top as a single layer, separated from the underlying stroma by a basal membrane (dotted line). The inserts show OSE cells in higher magnification, and primary cilia (arrows) on OSE cells visualized by IFM analysis with antibodies against acetylated α-tubulin (Acet.tub) and detyrosinated α-tubulin (Glu.tub). B-D) IHC analysis of ovarian tissue. OSE cells are characterized by being positive for the epithelial markers cytokeratin 8 (CK8) and cytokeratin 18 (CK18), and for the mesenchymal markers N-cadherin and vimentin, whereas they are E-cadherin negative. Nuclei are visualized with DAPI. DAPI, 4',6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; IHC, immunohistochemistry; OSE, ovarian surface epithelium.
Figure 2
Characterization of cell cultures of ovarian surface epithelium. A) Light microscopy (LM) images of wt and cancer OSE cells in cultures at sub-confluent and confluent stages. Anti-α-tubulin (α-tub) and phalloidin (F-actin) were applied to vizualize the cytoskeletal microtubules and actin, respectively, by IFM analysis, and anti-cytokeratin 8 (CK8) and anti-cytokeratin 18 (CK18) were applied to visualize the keratin components characteristic of human OSE cells. B) IFM analysis with primary antibodies against markers differentially expressed in wt and cancer OSE cells. See text for details. DNA was stained with DAPI. C) WB of wt and cancer OSE cells showing their expression of proteins characteristic for human OSE cells. EB1 was applied as loading control. DAPI, 4′,6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; OSE, ovarian surface epithelium; WB, western blot; wt, wild type.
Figure 3
Characterization of primary cilia formation in OSE cell cultures. Wild type (wt) and cancer OSE cells were grown to confluence followed by serum depletion for 72 hours to induce growth arrest and formation of primary cilia (arrows). Microtubules of the ciliary axoneme were detected with anti-acetylated α-tubulin (Acet.tub) and anti-detyrosinated α-tubulin (Glu.tub) in IFM analysis (A, B). The pericentriolar material of the centrosome and the centrioles were visualized with anti-pericentrin (PCTN) and anti-centrin (CT) antibodies, respectively. C) Cilia frequencies were determined, by IFM analysis with anti-Acet.tub and/or anti-Glu.tub, antibodies, as the number of ciliated cells over the total cell number in sub-confluent cultures in the presence of serum (0 hour) and in confluent cultures serum-depleted for 48 or 72 hours. Error bars represent standard deviations. Data were tested for significance using one-way ANOVA. The level of significance was set at P < 0.001 (***). D) Localization of IFT20 and IFT88 was visualized by IFM in serum-depleted wt OSE cultures. Nuclei were stained with DAPI. E) WB analysis of IFT88 and IFT20 in wt and cancer OSE cells in sub-confluent cultures with serum (0 hour) and confluent cultures depleted for serum for six, 24, or 72 hours. α-Tubulin (α-tub) was applied as loading control. ANOVA, analysis of variance; DAPI, 4′,6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; OSE, ovarian surface epithelium; WB, western blot; wt, wild type.
Figure 4
wt and cancer OSE cells enter growth arrest upon serum depletion. Analysis of known cell cycle markers in sub-confluent cultures with serum (0 hour) and confluent cultures serum depleted for 48 and/or 72 hours. A, B) WB analysis showing protein expression of phosphorylated retinoblastoma protein (p-RB) in wt and cancer OSE cells and quantification of p-RB levels in serum depleted cultures relative to cultures with serum and with respect to the loading controls (β-actin). C, D) WB analysis showing protein expression of proliferating cell nuclear antigen (PCNA) in wt and cancer OSE cells and quantification of PCNA levels in serum depleted cultures relative to cultures with serum and with respect to the loading controls (α-tub). E-G) IFM analysis of wt and cancer OSE cells using anti-acetylated α-tubulin (Acet.tub) antibody to detect primary cilia (arrows), and anti-Ki67 antibody to visualize Ki67 expression of cycling cells. Nuclei were visualized with DAPI. Note that most cancer cells enter growth arrest upon serum starvation, as judged by the lack of nuclear Ki67 staining. DAPI, 4′,6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; OSE, ovarian surface epithelium; WB, western blot; wt, wild type.
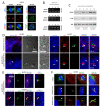
Figure 5
Hedgehog components and PDGFRα localize to OSE primary cilia. IFM analysis of serum-starved wt OSE cells using antibodies against the Hh components GLI2, SMO, and PTCH1 (A), or PDGFRα (D). Primary cilia (arrows) were detected with anti-acetylated α-tubulin (Acet.tub) antibody. B) WB analysis of wt and cancer OSE cells grown in the presence (0 hour) and or absence (72 hours) of serum. Blots were probed with antibodies against the full-length activator form of GLI2, GLI2(FL), or the C-terminally processed repressor form of GLI2, GLI(R). α-tubulin (α-tub) was applied as loading control. C) RT-PCR showing the expression level of the Hh responsive genes PTCH1 and GLI1 in cultures of wt and cancer OSE cells serum depleted for 72 hours. GAPDH was applied as an internal control. E) WB analysis of wt and cancer OSE cells grown in the presence (0 hour) or absence (72 hours) of serum. The PDGFRα antibody used recognizes two protein bands; #1 is the fully glycosylated form and #2 is the partly glycosylated form of the receptor. α-tub was applied as loading control. IFM, immunofluorescence microscopy; Hh, hedgehog; OSE, ovarian surface epithelium; WB, western blot; wt, wild type.
Figure 6
Aurora A localization and expression in wt and cancer OSE cells. IFM analysis of mitotic (A), interphase (D), and growth-arrested (D, E) wt and cancer OSE cells. Anti-pericentrin (PCNT), anti-EB3, anti-acetylated α-tubulin (Acet.tub), and anti-AURA were applied to detect the pericentriolar material (¤), centrioles (*), mitotic spindle and primary cilia (white arrows), and AURA, respectively. The rightmost column in (D) is a shifted overlay of the Acet.tub, AURA, and EB3 stainings. Cells in A, and rightmost column in E were fixed in mix-fix (see Methods for details), and cells in (D, E) were fixed in PFA + MeOH fix. B, C) AURA mRNA and protein levels were analyzed with RT-PCR (B) and WB (C), respectively, in sub-confluent cells with serum (0 hour) and in confluent cultures serum-depleted for 72 hours. GAPDH (B) and EB1 (C) were used as controls. Anti-phosphorylated retinoblastoma protein (p-RB) was included in WB analysis to verify that starved cells were in growth arrest (C). F) IFM analysis of checkpoint with forkhead-associated and ring finger domains (CHFR) localization in wt OSE cells during mitosis and in growth-arrested, serum-depleted cells. Cells were fixed in mix-fix and stained with antibodies as indicated. Primary cilia are marked with arrows and centrioles/basal bodies are marked with ¤. DNA was stained with DAPI. DAPI, 4′,6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; OSE, ovarian surface epithelium; WB, western blot; wt, wild type.
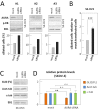
Figure 7
Inhibition of Aurora A and effect on ciliary formation in cancer OSE cells. A) Results of three independent siRNA knock-down experiments of AURA in SK-OV3 cells. Cells were grown to 60% confluency and transfected with scrambled oligonucleotides (mock) or siRNA targeting AURA mRNA. The cells were allowed to grow to confluence and 24 hours after transfection, medium was changed to serum-depleted medium. After 72 hours of incubation in serum-depleted medium, AURA protein levels were examined by WB analysis. Anti-phosphorylated retinoblastoma protein (p-RB) was included to explore the cell cycle stage. EB1 was applied as loading control. Percentages of ciliated cells were determined by IFM analysis with primary antibodies against acetylated α-tubulin, detyrosinated α-tubulin, and DAPI nuclear staining as the number of ciliated cells over the total cell number. Error bars represent standard deviations. B) Bar graph representation of the level of ciliated SK-OV3 cells in AURA siRNA transfected cells relative to mock transfected cells. The percentage of ciliated cells in mock transfected cells was set to one. Data were tested for significance using Student’s t-test. The level of significance was set at P < 0.001 (***). C) Protein levels of the full length activator form of GLI2, GLI2 (FL), acetylated α-tubulin (Acet.tub), and α-tubulin (α-tub) in mock and AURA siRNA transfected SK-OV3 cells serum-depleted for 72 hours. D) Bar graph representation of the relative protein levels in AURA siRNA transfected SK-OV3 cells compared to mock transfected cells. The protein level in mock transfected cells was set to one. Data were tested for significance using Student’s t-test. The effect of AURA siRNA treatment on Acet.tub and α-tub protein levels was found to be not significant (n.s.), whereas the treatment had a significant effect on GLI2 (FL), P < 0.01 (**). DAPI, 4‘,6-diamidino-2-phenylindole; IFM, immunofluorescence microscopy; OSE, ovarian surface epithelium; WB, western blot.
Similar articles
- Expression and action of transforming growth factor alpha in normal ovarian surface epithelium and ovarian cancer.
Doraiswamy V, Parrott JA, Skinner MK. Doraiswamy V, et al. Biol Reprod. 2000 Sep;63(3):789-96. doi: 10.1095/biolreprod63.3.789. Biol Reprod. 2000. PMID: 10952922 - Coexpression of hepatocyte growth factor-Met: an early step in ovarian carcinogenesis?
Wong AS, Pelech SL, Woo MM, Yim G, Rosen B, Ehlen T, Leung PC, Auersperg N. Wong AS, et al. Oncogene. 2001 Mar 15;20(11):1318-28. doi: 10.1038/sj.onc.1204253. Oncogene. 2001. PMID: 11313876 - PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts.
Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. Schneider L, et al. Curr Biol. 2005 Oct 25;15(20):1861-6. doi: 10.1016/j.cub.2005.09.012. Curr Biol. 2005. PMID: 16243034 - Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo-Oogenesis or Ovarian Tumorigenesis.
Xu J, Zheng T, Hong W, Ye H, Hu C, Zheng Y. Xu J, et al. Cell Physiol Biochem. 2018;50(1):214-232. doi: 10.1159/000494001. Epub 2018 Oct 18. Cell Physiol Biochem. 2018. PMID: 30336465 Review. - Primary cilium: an elaborate structure that blocks cell division?
Ke YN, Yang WX. Ke YN, et al. Gene. 2014 Sep 1;547(2):175-85. doi: 10.1016/j.gene.2014.06.050. Epub 2014 Jun 24. Gene. 2014. PMID: 24971504 Review.
Cited by
- Neoexpression of a functional primary cilium in colorectal cancer cells.
Sénicourt B, Boudjadi S, Carrier JC, Beaulieu JF. Sénicourt B, et al. Heliyon. 2016 May 11;2(5):e00109. doi: 10.1016/j.heliyon.2016.e00109. eCollection 2016 May. Heliyon. 2016. PMID: 27441280 Free PMC article. - Primary cilia contribute to the aggressiveness of atypical teratoid/rhabdoid tumors.
Blümel L, Qin N, Berlandi J, Paisana E, Cascão R, Custódia C, Pauck D, Picard D, Langini M, Stühler K, Meyer FD, Göbbels S, Malzkorn B, Liebau MC, Barata JT, Jeibmann A, Kerl K, Erkek S, Kool M, Pfister SM, Johann PD, Frühwald MC, Borkhardt A, Reifenberger G, Faria CC, Fischer U, Hasselblatt M, Bartl J, Remke M. Blümel L, et al. Cell Death Dis. 2022 Sep 20;13(9):806. doi: 10.1038/s41419-022-05243-4. Cell Death Dis. 2022. PMID: 36127323 Free PMC article. - Cilia and Cancer: From Molecular Genetics to Therapeutic Strategies.
Carotenuto P, Gradilone SA, Franco B. Carotenuto P, et al. Genes (Basel). 2023 Jul 11;14(7):1428. doi: 10.3390/genes14071428. Genes (Basel). 2023. PMID: 37510333 Free PMC article. Review. - Illumination of understudied ciliary kinases.
Flax RG, Rosston P, Rocha C, Anderson B, Capener JL, Durcan TM, Drewry DH, Prinos P, Axtman AD. Flax RG, et al. Front Mol Biosci. 2024 Mar 8;11:1352781. doi: 10.3389/fmolb.2024.1352781. eCollection 2024. Front Mol Biosci. 2024. PMID: 38523660 Free PMC article. Review. - Mechanisms for nonmitotic activation of Aurora-A at cilia.
Korobeynikov V, Deneka AY, Golemis EA. Korobeynikov V, et al. Biochem Soc Trans. 2017 Feb 8;45(1):37-49. doi: 10.1042/BST20160142. Biochem Soc Trans. 2017. PMID: 28202658 Free PMC article. Review.
References
- Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007;82:751–770. - PubMed
- Aoki Y, Kawada N, Tanaka K. Early form of ovarian cancer originating in inclusion cysts. A case report. J Reprod Med. 2000;45:159–161. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous