DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes - PubMed (original) (raw)
DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes
Hui-Chun Ku et al. PLoS One. 2013.
Abstract
Apart from the antihyperglycemic effects, DPP4 inhibitors and GLP-1 molecules are involved in the preservation of cardiac functions. We have demonstrated that DPP4-deficient rats possess resistance to endotoxemia and ischemia/reperfusion stress. However, whether the decrease of DPP4 activity simply augmented the GLP-1 signaling or that such decrease resulted in a change of cellular function remain unclear. Accordingly, we investigated the responses of H(2)O(2)-induced oxidative stress in adult wild-type and DPP4-deficient rats isolated cardiomyocytes. The coadministration of GLP-1 or DPP4 inhibitor was also performed to define the mechanisms. Cell viability, ROS concentration, catalase activity, glucose uptake, prosurvival, proapoptotic signaling, and contractile function were examined after cells exposed to H(2)O(2). DPP4-deficient cardiomyocytes were found to be resistant to H(2)O(2)-induced cell death via activating AKT signaling, enhancing glucose uptake, preserving catalase activity, diminishing ROS level and proapoptotic signaling. GLP-1 concentration-dependently improved cell viability in wild-type cardiomyocyte against ROS stress, and the ceiling response concentration (200 nM) was chosen for studies. GLP-1 was shown to decrease H(2)O(2)-induced cell death by its receptor-dependent AKT pathway in wild-type cardiomyocytes, but failed to cause further activation of AKT in DPP4-deficient cardiomyocytes. Acute treatment of DPP4 inhibitor only augmented the protective effect of low dose GLP-1, but failed to alter fuel utilization or ameliorate cell viability in wild-type cardiomyocytes after H(2)O(2) exposure. The improvement of cell viability after H(2)O(2) exposure was correlated with the alleviation of cellular contractile dysfunction in both DPP4-deficient and GLP-1 treated wild-type cardiomyocytes. These findings demonstrated that GLP-1 receptor-dependent pathway is important and exert protective effect in wild-type cardiomyocyte. Long term loss of DPP4 activity increased the capability against ROS stress, which was more than GLP-1 dependent pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
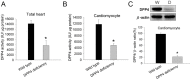
Figure 1. Measurement of DPP4 activity and protein expression.
DPP4 activity of (A) total heart and (B) cardiomyocyte protein were measured in wild-type and DPP4-deficient rats. (C)DPP4 expression was measured in two kinds of cardiomoyocytes by using western blot. W indicates wild-type, and DPP4 indicates DPP4 deficiency. (n = 5) *p<0.05 vs. wild-type.
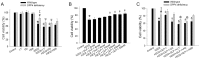
Figure 2. Effects of H2O2 treatment on cell viability in wild-type and DPP4-deficient cardiomyocytes.
(A) Cardiomyocytes isolated from two kinds of rats underwent H2O2 (300 µM) treatment for an hour. (B) GLP-1 concentration ranging from 20 nM to 500 nM was preincubated for an hour before H2O2 administration in wild-type cardiomyocyte. (C) GLP-1 (200 nM) was added for an hour before H2O2 exposured in both wild-type and DPP4-deficient cardiomyocytes. Antagonists of the GLP-1 receptor (Ex(9–39); 150 nM), PI3K (LY294002; 15 µM), MEK (PD98059; 15 µM), and PKA (H89; 20 µM) were used for 30 min before the addition of GLP-1. Cell viability was tested via MTT assay. (n = 5). *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2, Ψp<0.05 vs. DPP4-deficient H2O2, &p<0.05 vs. wild-type H2O2 + GLP-1, @p<0.05 vs. DPP4-deficient H2O2 + GLP-1.
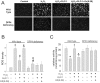
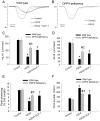
Figure 3. Effects of H2O2 treatment on ROS concentration and catalase activity in wild-type and DPP4-deficient cardiomyocytes.
Cardiomyocytes isolated from two kinds of rats underwent H2O2 treatment in the presence or absence of GLP-1 or GLP-1 receptor antagonist, ex(9–39). (A) Original microscopy photos were reported for ROS density, and (B) results of densitometry. (n = 3) (C) Catalase activity was reported. (n = 5) *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2, &p<0.05 vs. wild-type H2O2 + GLP-1.
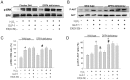
Figure 4. Effects of H2O2 treatment on Bax, Bcl-2 level, and caspase-3 activity in wild-type and DPP4-deficient cardiomyocytes, with or without GLP-1 or GLP-1 receptor antagonist cotreatment.
(A) Original western blots of Bax and Bcl-2 were reported. (B) Ratios of Bax to Bcl-2, and (C) Caspase-3 activity were reported. (n = 4) *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2, Ψp<0.05 vs. DPP4-deficient H2O2, &p<0.05 vs. wild-type H2O2 + GLP-1.
Figure 5. Effects of H2O2 treatment on ERK and AKT phosphorylation in wild-type and DPP4-deficient cardiomyocytes, with or without GLP-1 or GLP-1 receptor antagonist cotreatment.
(A) Original western blots of ERK and p-ERK were reported. (C) Ratios of p-ERK/ERK were shown. (B) Original western blots of AKT and p-AKT were reported. (D) Ratios of p-AKT/AKT were shown. (n = 3–4) *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2, &p<0.05 vs. wild-type H2O2 + GLP-1.
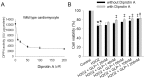
Figure 6. Effects of Diprotin A on cell viability after H2O2 treatment in wild-type cardiomyocyte.
(A) DPP4 activity was measured in Diprotin A-treated wild-type cardiomyocyte. (B) Cardiomyocytes isolated from wild-type rats underwent H2O2 (300 µM) treatment for an hour in the absence or presence of Diprotin A (50 µM, 90 min prior to H2O2 treatment). GLP-1 was added for 60 min before the addition of H2O2. (n = 4) *p<0.05 vs. wild-type control, ‡p<0.05 vs. wild-type H2O2.
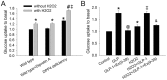
Figure 7. Effects of H2O2 treatment on glucose uptake in wild-type and DPP4-deficient cardiomyocytes.
(A) Glucose uptake was measured in wild-type, wild-type + Diprotin A, and DPP4-deficient cardiomyocytes with or without H2O2 cotreatment. (B) Wild-type cardiomyocytes were treated with GLP-1 or GLP-1 + ex(9–39) in the absence or presence of H2O2, and along with glucose uptake measurement (n = 4). *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2, &p<0.05 vs. wild-type H2O2 + GLP-1, @p<0.05 vs. wild-type + GLP-1.
Figure 8. Effects of H2O2 treatment on contractile function in wild-type and DPP4-deficient cardiomyocytes.
Cardiomcyoytes contractile function were measured after H2O2 (300 µM) exposure for an hour. GLP-1 (200 nM) was preincubated for an hour before H2O2 administration in wild-type cardiomyocytes. (A and B) Representative recordings of cell shortening were reported in wild-type and DPP4-deficient cardiomyocytes. (C) Maximal velocities of shortening (+dL/dt), (D) maximal velocities of relengthening (−dL/dt), (E) cell length of peak shortening, and (F) time to peak shortening were calculated. n = 22–30 cells from three rats per group. *p<0.05 vs. wild-type control, #p<0.05 vs. DPP4-deficient control, ‡p<0.05 vs. wild-type H2O2.
Similar articles
- Potential Role of Dipeptidyl Peptidase-4 in Regulating Mitochondria and Oxidative Stress in Cardiomyocytes.
Lee SY, Wu ST, Du CX, Ku HC. Lee SY, et al. Cardiovasc Toxicol. 2024 Oct;24(10):1090-1104. doi: 10.1007/s12012-024-09884-z. Epub 2024 Jul 2. Cardiovasc Toxicol. 2024. PMID: 38955919 - GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats.
Ku HC, Chen WP, Su MJ. Ku HC, et al. Naunyn Schmiedebergs Arch Pharmacol. 2010 Dec;382(5-6):463-74. doi: 10.1007/s00210-010-0559-9. Epub 2010 Sep 18. Naunyn Schmiedebergs Arch Pharmacol. 2010. PMID: 20852989 - DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion.
Ku HC, Chen WP, Su MJ. Ku HC, et al. Naunyn Schmiedebergs Arch Pharmacol. 2011 Aug;384(2):197-207. doi: 10.1007/s00210-011-0665-3. Epub 2011 Jul 12. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21748358 - DPP4 deficiency preserved cardiac function in abdominal aortic banding rats.
Ku HC, Su MJ. Ku HC, et al. PLoS One. 2014 Jan 9;9(1):e85634. doi: 10.1371/journal.pone.0085634. eCollection 2014. PLoS One. 2014. PMID: 24416433 Free PMC article. - DPP4 as a Potential Candidate in Cardiovascular Disease.
Chen SY, Kong XQ, Zhang KF, Luo S, Wang F, Zhang JJ. Chen SY, et al. J Inflamm Res. 2022 Sep 16;15:5457-5469. doi: 10.2147/JIR.S380285. eCollection 2022. J Inflamm Res. 2022. PMID: 36147690 Free PMC article. Review.
Cited by
- DPP-4 inhibition by linagliptin prevents cardiac dysfunction and inflammation by targeting the Nlrp3/ASC inflammasome.
Birnbaum Y, Tran D, Bajaj M, Ye Y. Birnbaum Y, et al. Basic Res Cardiol. 2019 Aug 6;114(5):35. doi: 10.1007/s00395-019-0743-0. Basic Res Cardiol. 2019. PMID: 31388770 - Combined Treatment with Sodium-Glucose Cotransporter-2 Inhibitor (Canagliflozin) and Dipeptidyl Peptidase-4 Inhibitor (Teneligliptin) Alleviates NASH Progression in A Non-Diabetic Rat Model of Steatohepatitis.
Ozutsumi T, Namisaki T, Shimozato N, Kaji K, Tsuji Y, Kaya D, Fujinaga Y, Furukawa M, Nakanishi K, Sato S, Sawada Y, Saikawa S, Kitagawa K, Takaya H, Kawaratani H, Kitade M, Moriya K, Noguchi R, Akahane T, Mitoro A, Yoshiji H. Ozutsumi T, et al. Int J Mol Sci. 2020 Mar 21;21(6):2164. doi: 10.3390/ijms21062164. Int J Mol Sci. 2020. PMID: 32245205 Free PMC article. - Cardiovascular Safety and Benefits of Semaglutide in Patients With Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6.
Nauck MA, Quast DR. Nauck MA, et al. Front Endocrinol (Lausanne). 2021 Mar 29;12:645566. doi: 10.3389/fendo.2021.645566. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33854484 Free PMC article. Review. - MiR-25 protects cardiomyocytes against oxidative damage by targeting the mitochondrial calcium uniporter.
Pan L, Huang BJ, Ma XE, Wang SY, Feng J, Lv F, Liu Y, Liu Y, Li CM, Liang DD, Li J, Xu L, Chen YH. Pan L, et al. Int J Mol Sci. 2015 Mar 10;16(3):5420-33. doi: 10.3390/ijms16035420. Int J Mol Sci. 2015. PMID: 25764156 Free PMC article. - Pleiotropic, heart rate-independent cardioprotection by ivabradine.
Kleinbongard P, Gedik N, Witting P, Freedman B, Klöcker N, Heusch G. Kleinbongard P, et al. Br J Pharmacol. 2015 Sep;172(17):4380-90. doi: 10.1111/bph.13220. Epub 2015 Jul 21. Br J Pharmacol. 2015. PMID: 26076181 Free PMC article.
References
- Duez H, Cariou B, Staels B (2012) DPP-4 inhibitors in the treatment of type 2 diabetes. Biochem Pharmacol 83: 823–832. - PubMed
- Ravassa S, Zudaire A, Diez J (2012) GLP-1 and cardioprotection. From bench to bedside. Cardiovasc Res 94: 316–323. - PubMed
- Hui H, Nourparvar A, Zhao X, Perfetti R (2003) Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144: 1444–1455. - PubMed
- Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, et al. (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 30: 1407–1414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
The authors work was supported by the grant from National Science Council of Taiwan, ROC (NSC-100-2325-B-002-066). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous