Prostate cancer screening and the management of clinically localized disease - PubMed (original) (raw)
Review
Prostate cancer screening and the management of clinically localized disease
Timothy J Wilt et al. BMJ. 2013.
No abstract available
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare: HUA is co-principal investigator in several investigator led diagnostic and focal therapy trials in prostate cancer supported by the MRC (UK), National Institute for Health Research Health Technology Assessment programme, Pelican Cancer Foundation charity, and Prostate Cancer UK charity; HUA receives funding from USHIFU and Advanced Medical Diagnostics for clinical trials; HUA has received consultancy payments in the past from Steba Biotech and Oncura/GE Healthcare and payment for conference travel from USHIFU; TJW is a volunteer member of the US Preventive Services Task Force and chairman of the Prostate cancer Intervention Versus Observation Trial (PIVOT).
Figures
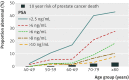
Fig 1 Impact of age on the proportion of men found to have an abnormal serum prostate specific antigen concentration depending on threshold used. The bars represent the 10 year risk of a prostate cancer related death in each age group
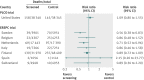
Fig 2 Forest plot showing the risk ratio with 95% confidence intervals from the two (of five) randomized prostate cancer screening trials judged to be at least “fair methodological quality” and of “low risk of bias” (PLCO and ERSPC). The vertical line represents no benefit
Fig 3 Benefits and harms of screening men aged 55-69 years* with a prostate specific antigen (PSA) test every 1-4 years for 10 years. Calculations rely on assumptions and are imprecise. Estimates should be considered in the full context of clinical decision making and used to stimulate shared decision making
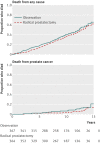
Fig 4 Kaplan-Meier curves showing death from any cause and death from prostate cancer in the Prostate Cancer Intervention versus Observation Trial (PIVOT) comparing radical prostatectomy with observation
Fig 5 Forest plots demonstrating subgroup effects (hazard ratio with 95% confidence intervals and P value for interaction) in the Prostate Cancer Intervention versus Observation Trial (PIVOT) comparing radical prostatectomy with observation. The vertical line indicates no effect. The size of the boxes indicates the weight of the effects
Comment in
- Antibiotic resistance and transrectal prostate biopsies.
Davies RJ, Stephenson BM, Llewelyn M, Carter AC, Kubiak E. Davies RJ, et al. BMJ. 2013 Feb 26;346:f1171. doi: 10.1136/bmj.f1171. BMJ. 2013. PMID: 23444435 No abstract available. - Prostate cancer screening.
Faigen M. Faigen M. Med J Aust. 2013 Nov 4;199(9):585. doi: 10.5694/mja13.10827. Med J Aust. 2013. PMID: 24182219 No abstract available.
Similar articles
- Prostate cancer screening.
Faigen M. Faigen M. Med J Aust. 2013 Nov 4;199(9):585. doi: 10.5694/mja13.10827. Med J Aust. 2013. PMID: 24182219 No abstract available. - Antibiotic resistance and transrectal prostate biopsies.
Davies RJ, Stephenson BM, Llewelyn M, Carter AC, Kubiak E. Davies RJ, et al. BMJ. 2013 Feb 26;346:f1171. doi: 10.1136/bmj.f1171. BMJ. 2013. PMID: 23444435 No abstract available. - Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.
Horwich A, Parker C, de Reijke T, Kataja V; ESMO Guidelines Working Group. Horwich A, et al. Ann Oncol. 2013 Oct;24 Suppl 6:vi106-14. doi: 10.1093/annonc/mdt208. Epub 2013 Jun 27. Ann Oncol. 2013. PMID: 23813930 No abstract available. - Prostate cancer: Determining optimal therapy of early-stage disease remains complicated.
Canes D. Canes D. Nat Rev Urol. 2016 Dec;13(12):703-704. doi: 10.1038/nrurol.2016.228. Epub 2016 Nov 8. Nat Rev Urol. 2016. PMID: 27824351 Review. No abstract available. - Prostate cancer: screening approaches.
Donovan JL, Martin RM, Neal DE, Hamdy FC. Donovan JL, et al. Br J Hosp Med (Lond). 2005 Nov;66(11):623-6. doi: 10.12968/hmed.2005.66.11.20023. Br J Hosp Med (Lond). 2005. PMID: 16308948 Review.
Cited by
- Radiation dose-response (a Bayesian model) in the radiotherapy of the localized prostatic adenocarcinoma: the reliability of PSA slope changes as a response surrogate endpoint.
Mohammadpour RA, Yazdani-Charati J, Faghani S, Alizadeh A, Barzegartahamtan M. Mohammadpour RA, et al. PeerJ. 2019 Jul 1;7:e7172. doi: 10.7717/peerj.7172. eCollection 2019. PeerJ. 2019. PMID: 31304057 Free PMC article. - Screening guidelines for non-AIDS defining cancers in HIV-infected individuals.
Mani D, Aboulafia DM. Mani D, et al. Curr Opin Oncol. 2013 Sep;25(5):518-25. doi: 10.1097/CCO.0b013e328363e04a. Curr Opin Oncol. 2013. PMID: 23942295 Free PMC article. Review. - Targeting aberrant sialylation and fucosylation in prostate cancer cells using potent metabolic inhibitors.
Orozco-Moreno M, Visser EA, Hodgson K, Hipgrave Ederveen AL, Bastian K, Goode EA, Öztürk Ö, Pijnenborg JFA, Eerden N, Moons SJ, Rossing E, Wang N, de Haan N, Büll C, Boltje TJ, Munkley J. Orozco-Moreno M, et al. Glycobiology. 2023 Dec 30;33(12):1155-1171. doi: 10.1093/glycob/cwad085. Glycobiology. 2023. PMID: 37847613 Free PMC article. - Comparative evaluation of urinary PCA3 and TMPRSS2: ERG scores and serum PHI in predicting prostate cancer aggressiveness.
Tallon L, Luangphakdy D, Ruffion A, Colombel M, Devonec M, Champetier D, Paparel P, Decaussin-Petrucci M, Perrin P, Vlaeminck-Guillem V. Tallon L, et al. Int J Mol Sci. 2014 Jul 30;15(8):13299-316. doi: 10.3390/ijms150813299. Int J Mol Sci. 2014. PMID: 25079439 Free PMC article. - Doctors' approaches to PSA testing and overdiagnosis in primary healthcare: a qualitative study.
Pickles K, Carter SM, Rychetnik L. Pickles K, et al. BMJ Open. 2015 Mar 17;5(3):e006367. doi: 10.1136/bmjopen-2014-006367. BMJ Open. 2015. PMID: 25783420 Free PMC article.
References
- Cancer Research UK. Prostate cancer statistics. 2012. www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/.
- Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst 2005;97:1132-7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical