Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma - PubMed (original) (raw)
Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma
Akiyo Tanabe et al. Horm Cancer. 2013 Apr.
Abstract
Choosing effective therapy for patients with malignant pheochromocytoma or paraganglioma (PPGL) is problematic and none of the options are curative. Although combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine (CVD) is an established treatment option, only a limited number of case series have been reported in the literature. To determine the efficacy of CVD in patients treated at Tokyo Women's Medical University. Retrospective review of patients treated with CVD between 1989 and 2012 was conducted. Demographics, clinical presentation, imaging, and laboratory reports were reviewed and analyzed. Efficacy of CVD was ascertained from the biochemical and tumor responses. Twenty-three patients fulfilled study criteria and 6 of these were excluded due to inadequate follow-up or discontinuance by poor general condition or adverse effects. Thus, 17 cases were included in the study. The age and duration of the disease before initiation of CVD were 54.7 ± 12.0 years and 9.1 ± 8.1 years, respectively. The follow-up period after initiation of CVD ranged from 12 to 192 months (median, 60 months). Complete or partial biochemical and/or partial tumor response was achieved in 47.1 % (responders). No significant biochemical or tumor response was seen in 23.5 % and deterioration in biochemical and tumor outcomes was seen in 29.4 % (non-responders). No patient showed complete biochemical and tumor responses. In responders, these effects were documented within 4 months after initiation of CVD with a progression-free survival of 31 to 60 months (median, 40 months). Age at the first diagnosis with PPGL was younger (P < 0.05) and the lag time to eventual diagnosis of malignant disease was longer (P < 0.05) in responders than those in non-responders. The responders had improvements in hypertension and impaired glucose tolerance. Although CVD chemotherapy is not curative for patients with malignant PPGL, it does provide approximately half of the patients with biochemical, tumor, and hypertension benefits.
Figures
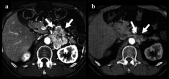
Fig. 1
CT before (a) and 2 years after (b) CVD chemotherapy in a patient who showed partial response in a tumor. Arrows show anterior margins of left retroperitoneal tumor mass
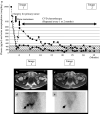
Fig. 2
Clinical course (a) of a patient who showed dissociation between catecholamine levels and MRI findings (b, c). Despite biochemical responses there was no tumor response as measured by MRI. While the findings on 123-I-MIBG (d, e) did correlate with the decline in catecholamine levels during treatment with CVD. The solid line shows urine norepinephrine (in microgram per day) and the dotted line shows plasma norepinephrine (in picogram per milliliter)
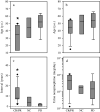
Fig. 3
Box plots (showing median, 25th and 75th percentiles, and an outlier as a closed circle) of age at the first diagnosis of pheochromocytoma (a), age at diagnosis of malignant disease (b), interval between the diagnosis of pheochromocytoma or paraganglioma to the detection of malignant disease (c) and urine norepinephrine level in the patients who were treated with CVD chemotherapy. CR/PR complete or partial response, NC no change, PD progressive disease. *P < 0.05 vs. PD
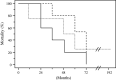
Fig. 4
Kaplan–Meier curves which are shown for overall survival in patients with complete or partial biochemical and/or partial tumor response (dashed line), no significant biochemical or tumor response (dotted line) and deterioration in biochemical and tumor responses (solid line)
Similar articles
- Controlling Tumor Progression with Cyclophosphamide, Vincristine, and Dacarbazine Treatment Improves Survival in Patients with Metastatic and Unresectable Malignant Pheochromocytomas/Paragangliomas.
Asai S, Katabami T, Tsuiki M, Tanaka Y, Naruse M. Asai S, et al. Horm Cancer. 2017 Apr;8(2):108-118. doi: 10.1007/s12672-017-0284-7. Epub 2017 Jan 20. Horm Cancer. 2017. PMID: 28108930 Free PMC article. - Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients.
Huang H, Abraham J, Hung E, Averbuch S, Merino M, Steinberg SM, Pacak K, Fojo T. Huang H, et al. Cancer. 2008 Oct 15;113(8):2020-8. doi: 10.1002/cncr.23812. Cancer. 2008. PMID: 18780317 Free PMC article. Clinical Trial. - Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis.
Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Niemeijer ND, et al. Clin Endocrinol (Oxf). 2014 Nov;81(5):642-51. doi: 10.1111/cen.12542. Epub 2014 Jul 30. Clin Endocrinol (Oxf). 2014. PMID: 25041164 Review. - Treatment of malignant phaeochromocytoma with a combination of cyclophosphamide, vincristine and dacarbazine: own experience and overview of the contemporary literature.
Deutschbein T, Fassnacht M, Weismann D, Reincke M, Mann K, Petersenn S. Deutschbein T, et al. Clin Endocrinol (Oxf). 2015 Jan;82(1):84-90. doi: 10.1111/cen.12590. Epub 2014 Oct 24. Clin Endocrinol (Oxf). 2015. PMID: 25143180 - [A case of metastatic pheochromocytoma with remarkable response to combination of cyclophosphamide, vincristine and dacarbazine].
Naoi Y, Tamaki Y, Ooka M, Tsukamoto F, Miyoshi Y, Tanji Y, Taguchi T, Noguchi S. Naoi Y, et al. Gan To Kagaku Ryoho. 2003 Jan;30(1):145-9. Gan To Kagaku Ryoho. 2003. PMID: 12557721 Review. Japanese.
Cited by
- Recent advances in the management of pheochromocytoma and paraganglioma.
Tanabe A, Naruse M. Tanabe A, et al. Hypertens Res. 2020 Nov;43(11):1141-1151. doi: 10.1038/s41440-020-0531-0. Epub 2020 Aug 11. Hypertens Res. 2020. PMID: 32778780 Review. - Treatment responses to antiangiogenetic therapy and chemotherapy in nonsecreting paraganglioma (PGL4) of urinary bladder with SDHB mutation: A case report.
Stigliano A, Lardo P, Cerquetti L, Aschelter AM, Matarazzo I, Capriotti G, Schiavi F, Marchetti P, Nardone MR, Petrangeli E, Toscano V. Stigliano A, et al. Medicine (Baltimore). 2018 Jul;97(30):e10904. doi: 10.1097/MD.0000000000010904. Medicine (Baltimore). 2018. PMID: 30045248 Free PMC article. - Synchronous liver metastasis at initial diagnosis of adrenal pheochromocytoma by CT: A case report.
Zhang Z, Jin X, Guo Y, Li X, Xu K, Jin G. Zhang Z, et al. Oncol Lett. 2024 May 17;28(1):329. doi: 10.3892/ol.2024.14462. eCollection 2024 Jul. Oncol Lett. 2024. PMID: 38807675 Free PMC article. - Model systems in SDHx-related pheochromocytoma/paraganglioma.
Takács-Vellai K, Farkas Z, Ősz F, Stewart GW. Takács-Vellai K, et al. Cancer Metastasis Rev. 2021 Dec;40(4):1177-1201. doi: 10.1007/s10555-021-10009-z. Epub 2021 Dec 27. Cancer Metastasis Rev. 2021. PMID: 34957538 Free PMC article. Review. - Primary malignant tumors of the adrenal glands.
Almeida MQ, Bezerra-Neto JE, Mendonça BB, Latronico AC, Fragoso MCBV. Almeida MQ, et al. Clinics (Sao Paulo). 2018 Dec 10;73(suppl 1):e756s. doi: 10.6061/clinics/2018/e756s. Clinics (Sao Paulo). 2018. PMID: 30540124 Free PMC article. Review.
References
- Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20:648–658. - PubMed
- Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S, Fitzgerald P. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27(25):4162–4168. doi: 10.1200/JCO.2008.21.3496. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical