The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS - PubMed (original) (raw)
. 2013 Jan 30;33(5):1927-39.
doi: 10.1523/JNEUROSCI.2080-12.2013.
Jacob A Sloane, Hu Li, Nurgul Aytan, Eustathia L Giannaris, Ella Zeldich, Jason D Hinman, Alpaslan Dedeoglu, Douglas L Rosene, Rashmi Bansal, Jennifer I Luebke, Makoto Kuro-o, Carmela R Abraham
Affiliations
- PMID: 23365232
- PMCID: PMC3711388
- DOI: 10.1523/JNEUROSCI.2080-12.2013
The antiaging protein Klotho enhances oligodendrocyte maturation and myelination of the CNS
Ci-Di Chen et al. J Neurosci. 2013.
Abstract
We have previously shown that myelin abnormalities characterize the normal aging process of the brain and that an age-associated reduction in Klotho is conserved across species. Predominantly generated in brain and kidney, Klotho overexpression extends life span, whereas loss of Klotho accelerates the development of aging-like phenotypes. Although the function of Klotho in brain is unknown, loss of Klotho expression leads to cognitive deficits. We found significant effects of Klotho on oligodendrocyte functions, including induced maturation of rat primary oligodendrocytic progenitor cells (OPCs) in vitro and myelination. Phosphoprotein analysis indicated that Klotho's downstream effects involve Akt and ERK signal pathways. Klotho increased OPC maturation, and inhibition of Akt or ERK function blocked this effect on OPCs. In vivo studies of Klotho knock-out mice and control littermates revealed that knock-out mice have a significant reduction in major myelin protein and gene expression. By immunohistochemistry, the number of total and mature oligodendrocytes was significantly lower in Klotho knock-out mice. Strikingly, at the ultrastructural level, Klotho knock-out mice exhibited significantly impaired myelination of the optic nerve and corpus callosum. These mice also displayed severe abnormalities at the nodes of Ranvier. To decipher the mechanisms by which Klotho affects oligodendrocytes, we used luciferase pathway reporters to identify the transcription factors involved. Together, these studies provide novel evidence for Klotho as a key player in myelin biology, which may thus be a useful therapeutic target in efforts to protect brain myelin against age-dependent changes and promote repair in multiple sclerosis.
Figures
Figure 1.
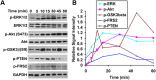
Phosphorylation kinetic analysis of Klotho-treated OPCs. A, OPCs were treated with Klotho for the times indicated, and the cell lysates were analyzed by Western blotting for protein phosphorylation of the proteins indicated. B, Kinetics of the signal intensity based on band densitometry as in A. Results represent the average of two independent experiments. Phosphorylated ERK1/2 and Akt are normalized to total ERK1/2 and Akt, respectively. All other phosphorylated proteins are normalized to GAPDH internal control.
Figure 2.
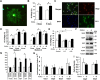
Klotho effects in vitro on primary OPCs. A–D, Klotho enhances oligodendrocyte maturation. Rat OPCs were treated with Klotho (+ Klotho) or with PBS (Control) for 3 d, and then immunostained for the mature oligodendrocyte marker O1 (green), and pan-oligodendrocyte marker Olig2 (red). A, A typical O1 staining of a differentiated oligodendrocyte. B, Statistical analysis of O1 and Olig2 staining of OPCs at 3 and 6 d treatment with Klotho or PBS. *Statistical significance (p < 0.005, t test). Error bars indicate SD. C, Immunostaining of OPCs with antibodies to the mature oligodendrocyte marker CC-1 (green) and pan-oligodendrocyte marker Olig2 (red). Cell nuclei were stained with DAPI (blue). White arrows in the merged image indicate nonoligodendrocytic cells, and yellow arrows indicate undifferentiated oligodendrocytes. D, Statistical analysis of the ratio of CC-1 to Olig2 staining of OPCs at 3, 6, and 8 d of treatment with Klotho or PBS in medium 1 (OPC culture medium containing CNTF and T3) or medium 2 (OPC culture medium containing CNTF, T3, and NT3). *Statistical significance (p < 0.005, t test). Error bars indicate SD. E, Klotho enhances OPC maturation via ERK and Akt signaling. Rat OPCs were treated with 0.5 μ
m
LY294002 (LY) (for Akt inhibition) or 1 μ
m
UO126 (UO) (for ERK inhibition) for 30 min before Klotho (KL) was added. OPCs were treated for 3 d and then immunostained as in D. Statistical analysis of the results is plotted. F, Klotho enhances major myelin proteins expression in rat OPCs. OPCs were treated with or without Klotho for 6 d in culture medium containing CNTF and T3 and blotted with the antibodies indicated with tubulin as loading control. G, The relative signal intensity based on band densitometry was plotted as relative percentage to control without Klotho treatment. Results represent the average of three to five independent experiments. All proteins are normalized to tubulin internal control. *Statistical significance (p < 0.005, t test). Error bars indicate SD. H, Klotho does not affect OPC cell viability. CellTiterGlo assay was used to assess Klotho's effect on OPC cell viability after 1–4 d of Klotho treatment. Error bars indicate SD. Results represent the average of six independent experiments. I, Klotho does not affect OPC cell proliferation. Cell numbers were assayed by crystal violet staining at day 2 and day 4 after Klotho treatment. Error bars indicate SD. Results represent the average of three independent experiments.
Figure 3.
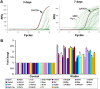
qRT-PCR confirmation of the effect of Klotho on OPC maturation. A, Representative qPCR results of differentiated OPCs for 3 and 7 d. The housekeeping gene GAPDH and the top two expressed genes, MBP and Plp1, are indicated. B, Fold change results of the upregulated genes by qPCR analysis of RNA from OPCs treated with Klotho for 7 d compared with control. OPCs were cultured in medium containing bFGF and PDGF for 3 d, and then changed to the differentiation medium with CNTF and T3 with or without Klotho for 7 d. *Statistical significance (p < 0.05, t test).
Figure 4.
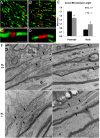
Klotho knock-out mice exhibit impaired myelination. A–L, The 4.5-week-old Klotho+/+, Klotho+/−, or Klotho−/− mice were processed for electron microscopy. Cross-sectional images show examples of myelination patterns for optic nerve (A-F) at 2900× (A, C, E) and 5800× (B, D, F) and corpus callosum (G–L) at 2900× (G, I, K) and 5800× (H, J, L). Scale bars: E, 2 μm; F, 500 nm. As, Astrocyte. M, The number of myelinated and unmyelinated axons were counted and graphed as percentage of myelinated fibers. Averages represent axonal counts analyzed from 3 to 6 different images. *Statistical significance (p < 0.0001, t test). Error bars indicate SD. ON, Optic nerve; CC, corpus callosum; SC, spinal cord. N, Klotho expression in CC, SC, and ON. Western blot analysis of Klotho from the lysates of 1.1- and 1.6-month-old control mice CC, SC, and ON tissues. O, Statistical analysis of Klotho expression in N with GAPDH as loading control. *Statistical significance (p < 0.05, t test). Error bars indicate SD. Sample size is n = 4 for each age. P, Major myelin proteins were largely reduced in Klotho knock-out mice. Western blot analysis of myelin markers from the brain lysates of 8-week-old Klotho knock-out (KL−/−) and control (KL+/+) mice. Q, Statistical analysis of the myelin markers in P with tubulin as control. *Statistical significance (p < 0.05, t test). Error bars indicate SD.
Figure 5.
Axonal microdomains are altered in Klotho−/− mice. Five sections each of corpus callosum from two wild-type and two homozygous Klotho−/− mice were stained for caspr (green) and β-IV spectrin (red). A, C, Wild-type mice have normal compact paranodal and nodal structure. B, D, Klotho−/− mice show an abundance of shorter paranodal segments and longer nodal segments (arrows). E, Measurements of nodal and paranodal length show a statistically significant decrease in paranodal length in Klotho−/− mice associated with a significant increase in nodal length. *Statistical significance (p < 0.001, t test). Scale bar, 2 μm. F, Electron microscopic longitudinal section analysis of nodes of Ranvier in corpus callosum from wild-type (KL+/+) and Klotho−/− (KL−/−) mice at 13,000×. Arrows indicate the junction between node and paranode. N, Node; P: paranode. Scale bars, 500 nm.
Figure 6.
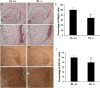
Immunohistochemistry and quantitative analysis of expression of Olig2 and GST-Pi in brain sections from 5-week-old Klotho+/+ and Klotho−/− mice. A–D, IHC images show examples of Olig2 nuclear staining patterns at 4× (A, B) and 20× (C, D). Scale bars: A, B, 200 μm; C, D, 50 μm. A, C, KL+/+. B, D, KL−/−. The fimbria region is outlined. Hb, Habenula. E, Cell counting of Olig2+ was performed as described in Materials and Methods. The number of Olig2+ cells in fimbria were counted and the percentage of Olig2+ cells in KL+/+ and KL−/− graphed. *Statistical significance (p < 0.01, t test). Error bars indicate SD. F–I, IHC of brain sections with antibodies to the mature oligodendrocyte marker GST-Pi at 4× (F, G) and 20× (H, I). Scale bars: F, G, 200 μm; H, I, 50 μm. J, Quantitation of GST-Pi+ cells as described in Materials and Methods. The GST-Pi+ cells in fimbria were counted and the percentage of GST-Pi+ cells in KL+/+ and KL−/− graphed. *Statistical significance (p < 0.05, t test). Error bars indicate SD.
Figure 7.
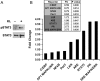
Transcription factors involved in Klotho regulation of OPCs. A, Western blot analysis of STAT3 phosphorylation upon Klotho treatment of OPC cells. B, Luciferase assay of luciferase reporters in OPC cells. OPCs were transfected with the reporter plasmids with Renilla luciferase and treated with or without Klotho for 24 h followed by luciferase assay. The fold change comparing Klotho treated to control is shown. p values indicate statistical significance by t test.
Similar articles
- Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve.
Ishibashi T, Lee PR, Baba H, Fields RD. Ishibashi T, et al. J Neurosci Res. 2009 Nov 15;87(15):3343-55. doi: 10.1002/jnr.22173. J Neurosci Res. 2009. PMID: 19598242 Free PMC article. - Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation.
Yang Y, Wang H, Zhang J, Luo F, Herrup K, Bibb JA, Lu R, Miller RH. Yang Y, et al. Dev Biol. 2013 Jun 15;378(2):94-106. doi: 10.1016/j.ydbio.2013.03.023. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583582 Free PMC article. - Conditional Deletion of the L-Type Calcium Channel Cav1.2 in NG2-Positive Cells Impairs Remyelination in Mice.
Santiago González DA, Cheli VT, Zamora NN, Lama TN, Spreuer V, Murphy GG, Paez PM. Santiago González DA, et al. J Neurosci. 2017 Oct 18;37(42):10038-10051. doi: 10.1523/JNEUROSCI.1787-17.2017. Epub 2017 Sep 12. J Neurosci. 2017. PMID: 28899915 Free PMC article. - The role of hyaluronan in myelination and remyelination after white matter injury.
Diao S, Xiao M, Chen C. Diao S, et al. Brain Res. 2021 Sep 1;1766:147522. doi: 10.1016/j.brainres.2021.147522. Epub 2021 May 16. Brain Res. 2021. PMID: 34010609 Review. - Seeing Is Believing: Myelin Dynamics in the Adult CNS.
Swire M, Ffrench-Constant C. Swire M, et al. Neuron. 2018 May 16;98(4):684-686. doi: 10.1016/j.neuron.2018.05.005. Neuron. 2018. PMID: 29772200 Review.
Cited by
- Oligodendrocyte progenitor cells in Alzheimer's disease: from physiology to pathology.
Zou P, Wu C, Liu TC, Duan R, Yang L. Zou P, et al. Transl Neurodegener. 2023 Nov 14;12(1):52. doi: 10.1186/s40035-023-00385-7. Transl Neurodegener. 2023. PMID: 37964328 Free PMC article. Review. - Demyelination and impaired oligodendrogenesis in the corpus callosum following lead exposure.
Liu LL, Emir U, Gu H, Sang LT, Sawiak SJ, Cannon JR, Du Y, Zheng W. Liu LL, et al. Toxicol Sci. 2024 Nov 1;202(1):123-141. doi: 10.1093/toxsci/kfae100. Toxicol Sci. 2024. PMID: 39150886 - Differential Expression of Klotho in the Brain and Spinal Cord is Associated with Total Antioxidant Capacity in Mice with Experimental Autoimmune Encephalomyelitis.
Emami Aleagha MS, Harirchian MH, Lavasani S, Javan M, Allameh A. Emami Aleagha MS, et al. J Mol Neurosci. 2018 Apr;64(4):543-550. doi: 10.1007/s12031-018-1058-6. Epub 2018 Mar 14. J Mol Neurosci. 2018. PMID: 29542092 - Long-Term Estrogen Receptor Beta Agonist Treatment Modifies the Hippocampal Transcriptome in Middle-Aged Ovariectomized Rats.
Sárvári M, Kalló I, Hrabovszky E, Solymosi N, Rodolosse A, Liposits Z. Sárvári M, et al. Front Cell Neurosci. 2016 Jun 10;10:149. doi: 10.3389/fncel.2016.00149. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27375434 Free PMC article. - Enhanced Expression of Secreted α-Klotho in the Hippocampus Alters Nesting Behavior and Memory Formation in Mice.
Li D, Jing D, Liu Z, Chen Y, Huang F, Behnisch T. Li D, et al. Front Cell Neurosci. 2019 Apr 2;13:133. doi: 10.3389/fncel.2019.00133. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31001090 Free PMC article.
References
- Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. - PubMed
- Bansal R, Magge S, Winkler S. Specific inhibitor of FGF receptor signaling: FGF-2-mediated effects on proliferation, differentiation, and MAPK activation are inhibited by PD173074 in oligodendrocyte-lineage cells. J Neurosci Res. 2003;74:486–493. - PubMed
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15:314–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous