Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12 - PubMed (original) (raw)
Neurokinin-1 receptor agonists bias therapeutic dendritic cells to induce type 1 immunity by licensing host dendritic cells to produce IL-12
Brian M Janelsins et al. Blood. 2013.
Abstract
Substance-P and hemokinin-1 are proinflammatory neuropeptides with potential to promote type 1 immunity through agonistic binding to neurokinin-1 receptor (NK1R). Dendritic cells (DCs) are professional antigen-presenting cells that initiate and regulate the outcome of innate and adaptive immune responses. Immunostimulatory DCs are highly desired for the development of positive immunization techniques. DCs express functional NK1R; however, regardless of their potential DC-stimulatory function, the ability of NK1R agonists to promote immunostimulatory DCs remains unexplored. Here, we demonstrate that NK1R signaling activates therapeutic DCs capable of biasing type 1 immunity by inhibition of interleukin-10 (IL-10) synthesis and secretion, without affecting their low levels of IL-12 production. The potent type 1 effector immune response observed following cutaneous administration of NK1R-signaled DCs required their homing in skin-draining lymph nodes (sDLNs) where they induced inflammation and licensed endogenous-conventional sDLN-resident and -recruited inflammatory DCs to secrete IL-12. Our data demonstrate that NK1R signaling promotes immunostimulatory DCs, and provide relevant insight into the mechanisms used by neuromediators to regulate innate and adaptive immune responses.
Figures
Figure 1
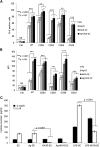
NK1R-DCs are mature DCs that do not secrete IL-10. (A-B) Expression of Ag- presenting (MHC class II, IAb), activation (CD83), and costimulatory (CD80, CD86, and CD40) molecules by untreated DCs, Ag-DCs, NK1R-DCs, and Ag-NK1R-DCs, analyzed by flow cytometry. (A) Percentages of positive DCs, (B) intensity of expression of each marker, as MFI. Means ± 1 SD of 3 independent experiments are shown. (C) Secretion of IL-12p70 and IL-10 (analyzed by ELISA) by untreated DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs (the latter cultured with or without LPS). Means ± 1 SD from 3 independent experiments are displayed. MFI, mean fluorescence intensity.
Figure 2
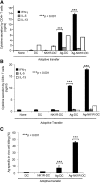
NK1R signaling increases the capacity of BMDCs to elicit type 1 immunity. (A-B) Cytokine secretion (analyzed by ELISA) of splenic (A) CD4 or (B) CD8 T cells obtained from C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. (C) Analysis of Ag-specific CTL function by in vivo killing assays, 7 days after footpad immunization (1 dose) of C57BL/6 mice with untreated DCs, NK1R-DCs, Ag-DCs or Ag-NK1R-DCs. (A-B) Means ± 1 SD of triplicates from 1 of 3 experiments is shown. (C) Means ± 1 SD from 6 mice per experimental group are shown.
Figure 3
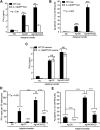
Type 1 immunity elicited by footpad immunization with NK1R-DCs requires IL-12p70 released by host DCs. (A) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells from WT or IL-12p35KO C57BL/6 mice 7 days after footpad immunization (1 dose) with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Percentage of Ag-specific CTL function (in vivo killing assays) in WT or IL-12p35KO C57BL/6 mice, 7 days following footpad immunization (1 dose) as described in panel A. (C) Secretion of IFN-γ (analyzed by ELISA) by splenic CD4 T cells isolated from WT mice, 7 days after footpad immunization (1 dose) with WT or IL-12p35KO untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (D) Assessment of IFN-γ–secreting CD4 T cells (by ELISPOT) from spleens of CD11c-DTR mice, depleted or not of host DCs with DT injection 1 day before immunization, and analyzed 7 days following footpad immunization. (E) Percentage of Ag-specific CTL activity (determined using in vivo killing assays) in CD11c-DTR mice treated or not with DT 1 day before administration of untreated DCs, Ag-DCs, or Ag-NK1R-DCs, analyzed 7 days after vaccination. Means ± 1 SD from 5 mice per group are shown. NS, not significant difference.
Figure 4
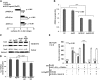
NK1R-DCs downregulate CREB1/TORC2 nuclear translocation resulting in decreased IL-10 and increased IL-12p70 secretion by LN-DCs. (A) Expression of luciferase (relative luminic units) by BMDCs transfected with plasmid DNA encoding the reporter protein under the transcriptional control of CREB1-, NFκB-binding enhancer elements, or the CMV promoter. BMDCs were left untreated (control) or cultured with the NK1R agonist SarSP, LPS, or agonist CD40 Ab. (B) Expression of CREB transcripts analyzed by quantitative RT-PCR in untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Means ± 1 SD of triplicates of 1 representative experiment of 2 are illustrated. (C-D) Negative regulation of TORC2 translocation in BMDCs nuclei by NK1R signaling. (C) Western blot analysis of TORC2 content in cytoplasmic and nuclear protein lysates prepared from untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs, resolved by SDS-PAGE, and probed for TORC2. (D) Percentages TORC2 translocation based on normalized density values of 3 independent experiments. (E) Secretion of IL-12p70 and IL-10 (by ELISA) in 24-hour supernatants of cocultures of (1) IL-12p35KO BMDCs, left untreated (DC), NK1R-DCs, the latter transduced or not with RAd–IL-10, and (2) DCs freshly-isolated from LNs (LN-DC) stimulated or not (control) with IFN-γ and agonist CD40 Ab. (A-E) Means ± 1 SD of triplicates from 1 representative experiment of 3 are shown.
Figure 5
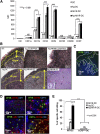
NK1R-signaled BMDCs are required to home in sDLNs to promote type 1 immunity. (A) Flow cytometric analysis of the expression of homotypic adhesion molecules and the chemokine receptor CCR7 by untreated-DCs, NK1R-DCs, Ag-DCs, and Ag-NK1R-DCs. Mean ± 1 SD of MFI of 3 independent experiments are illustrated. (B) Microscopic analysis (H&E, PAS) of sDLN, 24 hours after subcutaneous immunization with WT or CCR7KO BMDCs untreated (DC), NK1R-DCs, or Ag-NK1R-DCs. Paracortical areas (dotted lines) are defined by the presence of PAS+ high endothelial veins (arrowheads, inset). H&E and PAS, ×200. Inset, ×500. (C) Identification by fluorescence microscopy of CFSE+ NK1R-DCs 24 hours after subcutaneous injection and homed in the subcapsular and paracortical areas of sDLN (dotted lines). Nuclei were stained blue with DAPI, ×200. (D) Colocalization of CFSE+ Ag-NK1R-DCs (injected subcutaneously, 24 hours prior), and endogenous CD11c+CD11b+, CD11c+CD11b−, and CD11c+Ly6C+ DCs in sDLNs (top and bottom left panels) and CD3+ T cells (bottom right panel). Fluorescence microscopy, ×100 and ×200. (E) Comparative analysis of CTL function in WT mice skin-vaccinated with WT or CCR7KO, NK1R-DCs, Ag-DCs, or Ag-NK1R-DCs. The Ag-specific CTL activity was measured 7 days after immunization by in vivo killing assays. Means ± 1 SD from 5 mice per group are shown. CFSE, carboxyfluorescein diacetate succinimidyl ester; DAPI, 4,6 diamidino-2-phenylindole.
Figure 6
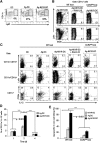
In sDLN, secretion of IL-12 is limited to endogenous DCs and induction of CTL function requires recruitment of inflammatory DCs. (A) Percentages of CD11c+Ly6C+ inflammatory DCs (flow cytometry) in sDLN after skin immunization with untreated DCs, Ag-DCs, or Ag-NK1R-DCs. (B) Further characterization (flow cytometry) of CD11c+Ly6C+ DCs coexpressing TNF-α or iNOS in sDLNs, 2 days after administration of WT Ag-NK1R-DCs, transduced or not with RAd–IL-10, to WT or CCR2KO mice. (C) Identification (flow cytometry) in sDLNs of the DC subsets secreting IL-12p40/70, 2 days after skin immunization of WT or CCR2KO mice with WT untreated-DCs, Ag-DCs, or Ag-NK1R-DCs, the latter transduced or not with RAd–IL-10. (D) Flow cytometric analysis of IL-12p70 kinetics in sDLNs following subcutaneous administration of Ag-DCs or Ag-NK1R-DCs. (E) Comparative analysis of the CTL function (in vivo killing assays), 7 days after skin vaccination of WT or CCR2KO mice with WT, Ag-DCs, NK1R-DCs, or Ag-NK1R-DCs. (A-C) Results from 1 representative of 3 independent experiments are shown. (D) Means ± 1 SD of 5 mice per experimental group are shown.
Figure 7
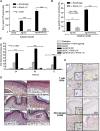
The ability of NK1R-signaled BMDCs to elicit robust type 1 immunity is abrogated by transgenic IL-10 secretion. (A) Quantification of splenic IFN-γ–secreting CD4 T cells (ELISPOT) in mouse immunized subcutaneously with Ag-DCs or Ag-NK1R-DC, transduced with RAd–IL-10 or with control RAd-Empty. Means ± 1 SD of 3 independent experiments are shown. (B) Ag-specific CTL function (in vivo killing assay), analyzed 7 days after subcutaneous immunization with Ag-DCs or Ag-NK1R-DCs, transfected with RAd–IL-10 or control RAd-Empty. Means ± 1 SD from 5 mice per group are shown. (C) Percentage of ear thickness increase (DTH assay) assessed in mice sensitized with 1 dose of Ag-DCs or Ag-NK1R-DCs, transduced or not with RAd–IL-10. Mean ± 1 SD from 5 mice per group are shown. (D) Histologic analysis of the leukocyte infiltration in the ear skin from the area of DTH elicitation. H&E, ×200. (E) Detection of the T-cell and macrophage infiltrates in the ear skin from the area of DTH elicitation. Peroxidase, ×200. Insets, ×500.
Comment in
- Harnessing DCs by substance P.
Takashima A. Takashima A. Blood. 2013 Apr 11;121(15):2815-6. doi: 10.1182/blood-2013-02-483354. Blood. 2013. PMID: 23580632
Similar articles
- Proinflammatory tachykinins that signal through the neurokinin 1 receptor promote survival of dendritic cells and potent cellular immunity.
Janelsins BM, Mathers AR, Tkacheva OA, Erdos G, Shufesky WJ, Morelli AE, Larregina AT. Janelsins BM, et al. Blood. 2009 Mar 26;113(13):3017-26. doi: 10.1182/blood-2008-06-163121. Epub 2008 Nov 5. Blood. 2009. PMID: 18987361 Free PMC article. - In vivo signaling through the neurokinin 1 receptor favors transgene expression by Langerhans cells and promotes the generation of Th1- and Tc1-biased immune responses.
Mathers AR, Tckacheva OA, Janelsins BM, Shufesky WJ, Morelli AE, Larregina AT. Mathers AR, et al. J Immunol. 2007 Jun 1;178(11):7006-17. doi: 10.4049/jimmunol.178.11.7006. J Immunol. 2007. PMID: 17513750 - Harnessing DCs by substance P.
Takashima A. Takashima A. Blood. 2013 Apr 11;121(15):2815-6. doi: 10.1182/blood-2013-02-483354. Blood. 2013. PMID: 23580632 - Dendritic cells: arbiters of immunity and immunological tolerance.
Lewis KL, Reizis B. Lewis KL, et al. Cold Spring Harb Perspect Biol. 2012 Aug 1;4(8):a007401. doi: 10.1101/cshperspect.a007401. Cold Spring Harb Perspect Biol. 2012. PMID: 22855722 Free PMC article. Review. - Innate-adaptive crosstalk: how dendritic cells shape immune responses in the CNS.
Clarkson BD, Héninger E, Harris MG, Lee J, Sandor M, Fabry Z. Clarkson BD, et al. Adv Exp Med Biol. 2012;946:309-33. doi: 10.1007/978-1-4614-0106-3_18. Adv Exp Med Biol. 2012. PMID: 21948376 Free PMC article. Review.
Cited by
- Neurotransmitters: promising immune modulators in the tumor microenvironment.
Xiao L, Li X, Fang C, Yu J, Chen T. Xiao L, et al. Front Immunol. 2023 May 5;14:1118637. doi: 10.3389/fimmu.2023.1118637. eCollection 2023. Front Immunol. 2023. PMID: 37215113 Free PMC article. Review. - Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis.
Suvas S. Suvas S. J Immunol. 2017 Sep 1;199(5):1543-1552. doi: 10.4049/jimmunol.1601751. J Immunol. 2017. PMID: 28827386 Free PMC article. Review. - Loss of Neurokinin-1 Receptor Alters Ocular Surface Homeostasis and Promotes an Early Development of Herpes Stromal Keratitis.
Gaddipati S, Rao P, Jerome AD, Burugula BB, Gerard NP, Suvas S. Gaddipati S, et al. J Immunol. 2016 Nov 15;197(10):4021-4033. doi: 10.4049/jimmunol.1600836. Epub 2016 Oct 17. J Immunol. 2016. PMID: 27798158 Free PMC article. - The Eye Sees Eye to Eye With the Immune System: The 2019 Proctor Lecture.
Niederkorn JY. Niederkorn JY. Invest Ophthalmol Vis Sci. 2019 Oct 1;60(13):4489-4495. doi: 10.1167/iovs.19-28632. Invest Ophthalmol Vis Sci. 2019. PMID: 31661549 Free PMC article. No abstract available. - Immunomodulatory Role of Neuropeptides in the Cornea.
Puri S, Kenyon BM, Hamrah P. Puri S, et al. Biomedicines. 2022 Aug 16;10(8):1985. doi: 10.3390/biomedicines10081985. Biomedicines. 2022. PMID: 36009532 Free PMC article. Review.
References
- Blalock JE, Smith EM. Conceptual development of the immune system as a sixth sense. Brain Behav Immun. 2007;21(1):23–33. - PubMed
- Andersson J. The inflammatory reflex—introduction. J Intern Med. 2005;257(2):122–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases