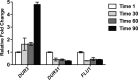Candida albicans flu1-mediated efflux of salivary histatin 5 reduces its cytosolic concentration and fungicidal activity - PubMed (original) (raw)
Candida albicans flu1-mediated efflux of salivary histatin 5 reduces its cytosolic concentration and fungicidal activity
Rui Li et al. Antimicrob Agents Chemother. 2013 Apr.
Abstract
Histatin 5 (Hst 5) is a salivary human antimicrobial peptide that is toxic to the opportunistic yeast Candida albicans. Fungicidal activity of Hst 5 requires intracellular translocation and accumulation to a threshold concentration for it to disrupt cellular processes. Previously, we observed that total cytosolic levels of Hst 5 were gradually reduced from intact cells, suggesting that C. albicans possesses a transport mechanism for efflux of Hst 5. Since we identified C. albicans polyamine transporters responsible for Hst 5 uptake, we hypothesized that one or more polyamine efflux transporters may be involved in the efflux of Hst 5. C. albicans FLU1 and TPO2 were found to be the closest homologs of Saccharomyces cerevisiae TPO1, which encodes a major spermidine efflux transporter, indicating that the products of these two genes may be involved in efflux of Hst 5. We found that flu1Δ/Δ cells, but not tpo2Δ/Δ cells, had significant reductions in their rates of Hst 5 efflux and had significantly higher cytoplasmic Hst 5 and Hst 5 susceptibilities than did the wild type. We also found that flu1Δ/Δ cells had reduced biofilm formation compared to wild-type cells in the presence of Hst 5. Transcriptional levels of FLU1 were not altered over the course of treatment with Hst 5; therefore, Hst 5 is not likely to induce FLU1 gene overexpression as a potential mechanism of resistance. Thus, Flu1, but not Tpo2, mediates efflux of Hst 5 and is responsible for reduction of its toxicity in C. albicans.
Figures
Fig 1
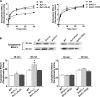
Phylogenetic analysis of C. albicans_TPO_ transporter family members and their relationship with Saccharomyces cerevisiae Tpo. Members of the C. albicans_TPO_ transporter family (
) are shown with TPO-homologous protein sequences from the yeast S. cerevisiae. C. albicans FLU1 (orf19.6577)- and TPO2 (orf19.7148)-encoded proteins have the closest phylogenetic relationship to S. cerevisiae polyamine excretion protein Tpo1, a spermidine efflux transporter.
Fig 2
Efflux of BHst 5 is reduced, resulting in increased cytoplasmic accumulation in the _C. albicans flu1_Δ/Δ strain. (A) C. albicans (wild-type, _flu1_Δ/Δ and _tpo2_Δ/Δ mutant, and _flu1_Δ/FLU1 and _tpo2_Δ/TPO2 restoration strains) were loaded with biotin-labeled Hst 5 (BHst 5) (31 μM) for 20 min and then resuspended in NaPB (pH 5) for 0 min, 2 min, 10 min, 20 min, and 30 min. Supernatants were collected at each time point and immunoblotted to detect extracellular concentrations of BHst 5 by densitometry for each time point. Only the _flu1_Δ/Δ mutant had significantly (P < 0.01) reduced BHst 5 in cell supernatants. (B) Cytosolic concentrations of BHst 5 were quantified following incubation of cells with BHst 5 (31 μM) for 1 min, 30 min, and 60 min. Cytoplasmic fractions were isolated at each time point, resolved using 12.5% SDS-PAGE, immunoblotted, and quantified as described above. Only the _flu1_Δ/Δ mutant had significantly (P < 0.05) increased cytosolic BHst 5 at both 30 min and 60 min compared to wild-type cells. Data shown are means from at least three independent experiments.
Fig 3
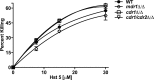
C. albicans drug transporter-deficient strains are not altered in sensitivity to Hst 5. C. albicans deletion mutants (_cdr1_Δ/Δ, _mdr1_Δ/Δ, and _cdr1/cdr2_Δ/Δ) were exposed to Hst 5 (7.5 μM to 31 μM) using microdilution plate assays, and percent killing was calculated. There was no significant difference of sensitivity to Hst 5 between wild-type and mutant strains.
Fig 4
Expression of C. albicans FLU1 is not altered following treatment of cells with Hst 5. Hst 5 (31 μM) was added to cultures at 0 min, and aliquots were removed for RNA extraction at 1, 30, 60, and 90 min as indicated. Transcriptional profiles of C. albicans DUR3, DUR31, and FLU1 were characterized by quantitative real-time RT-PCR, and expression levels were normalized to 18S RNA. Values for each gene are expressed as a change (_n_-fold) relative to the 1-min time point. Only DUR3 transcript levels were increased at 90 min compared to wild-type cells.
Fig 5
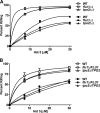
The _C. albicans flu1_Δ/Δ mutant has increased sensitivity to Hst 5. C. albicans deletion mutants (_flu1_Δ/Δ and _tpo2_Δ/Δ and restoration strains) were exposed to Hst 5 (7.5 μM to 31 μM) for 30 min at pH 5 (open circles) and pH 7 (filled circles) using microdilution plate assays, and percent killing was calculated. The _C. albicans flu1_Δ/Δ strain was found to be hypersensitive to Hst 5 under both neutral and acidic conditions, while the _tpo2_Δ/Δ strain did not differ from the parental WT strain in its sensitivity to Hst 5. (B) Restoration strains (_flu1_Δ/FLU1 and _tpo2_Δ/TPO2) had sensitivity to Hst 5 similar to that of the parental strains under both neutral and acidic conditions.
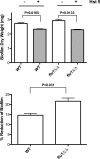
Fig 6
_C. albicans flu1_Δ/Δ biofilms have increased sensitivity to Hst 5. Biofilms were formed for 24 h in tissue culture plates using wild-type and _flu1_Δ/Δ cells, and then Hst 5 (60 μM) was added for 1 h and quenched with addition of fresh yeast nitrogen base (YNB) medium. Plates were incubated at 37°C for another 36 h, and then biofilms were removed mechanically and dry weights of cells per well were calculated. Reduction of biofilm formation in the presence of Hst 5 was detected in both the wild type (P = 0.0165) and the _flu1_Δ/Δ strain (P = 0.0133). The _flu1_Δ/Δ cells had a significantly higher percentage of biofilm reduction (21.6 ± 2.2) than did wild-type cells (14.6 ± 1.2).
Similar articles
- Histatin 5 uptake by Candida albicans utilizes polyamine transporters Dur3 and Dur31 proteins.
Kumar R, Chadha S, Saraswat D, Bajwa JS, Li RA, Conti HR, Edgerton M. Kumar R, et al. J Biol Chem. 2011 Dec 23;286(51):43748-43758. doi: 10.1074/jbc.M111.311175. Epub 2011 Oct 27. J Biol Chem. 2011. PMID: 22033918 Free PMC article. - Histatin 5 resistance of Candida glabrata can be reversed by insertion of Candida albicans polyamine transporter-encoding genes DUR3 and DUR31.
Tati S, Jang WS, Li R, Kumar R, Puri S, Edgerton M. Tati S, et al. PLoS One. 2013 Apr 22;8(4):e61480. doi: 10.1371/journal.pone.0061480. Print 2013. PLoS One. 2013. PMID: 23613860 Free PMC article. - A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole.
Calabrese D, Bille J, Sanglard D. Calabrese D, et al. Microbiology (Reading). 2000 Nov;146 ( Pt 11):2743-2754. doi: 10.1099/00221287-146-11-2743. Microbiology (Reading). 2000. PMID: 11065353 - How does it kill?: understanding the candidacidal mechanism of salivary histatin 5.
Puri S, Edgerton M. Puri S, et al. Eukaryot Cell. 2014 Aug;13(8):958-64. doi: 10.1128/EC.00095-14. Epub 2014 Jun 20. Eukaryot Cell. 2014. PMID: 24951439 Free PMC article. Review. - All about CDR transporters: Past, present, and future.
Prasad R, Balzi E, Banerjee A, Khandelwal NK. Prasad R, et al. Yeast. 2019 Apr;36(4):223-233. doi: 10.1002/yea.3356. Epub 2018 Oct 29. Yeast. 2019. PMID: 30192990 Review.
Cited by
- Histatin 5-spermidine conjugates have enhanced fungicidal activity and efficacy as a topical therapeutic for oral candidiasis.
Tati S, Li R, Puri S, Kumar R, Davidow P, Edgerton M. Tati S, et al. Antimicrob Agents Chemother. 2014;58(2):756-66. doi: 10.1128/AAC.01851-13. Epub 2013 Nov 18. Antimicrob Agents Chemother. 2014. PMID: 24247141 Free PMC article. - Oropharyngeal Candidiasis: Fungal Invasion and Epithelial Cell Responses.
Swidergall M, Filler SG. Swidergall M, et al. PLoS Pathog. 2017 Jan 12;13(1):e1006056. doi: 10.1371/journal.ppat.1006056. eCollection 2017 Jan. PLoS Pathog. 2017. PMID: 28081240 Free PMC article. Review. No abstract available. - Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota.
Höfs S, Mogavero S, Hube B. Höfs S, et al. J Microbiol. 2016 Mar;54(3):149-69. doi: 10.1007/s12275-016-5514-0. Epub 2016 Feb 27. J Microbiol. 2016. PMID: 26920876 Review. - Membrane Proteome-Wide Response to the Antifungal Drug Clotrimazole in Candida glabrata: Role of the Transcription Factor CgPdr1 and the Drug:H+ Antiporters CgTpo1_1 and CgTpo1_2.
Pais P, Costa C, Pires C, Shimizu K, Chibana H, Teixeira MC. Pais P, et al. Mol Cell Proteomics. 2016 Jan;15(1):57-72. doi: 10.1074/mcp.M114.045344. Epub 2015 Oct 28. Mol Cell Proteomics. 2016. PMID: 26512119 Free PMC article. - Mangiferin enhances the antifungal activities of caspofungin by destroying polyamine accumulation.
Shen J, Lu R, Cai Q, Fan L, Yan W, Zhu Z, Yang L, Cao Y. Shen J, et al. Virulence. 2021 Dec;12(1):217-230. doi: 10.1080/21505594.2020.1870079. Virulence. 2021. PMID: 33404349 Free PMC article.
References
- Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A, Barcelona Candidemia Project Study Group 2005. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 43:1829–1835 - PMC - PubMed
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172–1177 - PubMed
- Paphitou NI, Ostrosky-Zeichner L, Rex JH. 2005. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med. Mycol. 43:235–243 - PubMed
- Spellberg BJ, Filler SG, Edwards JE. 2006. Current treatment strategies for disseminated candidiasis. Clin. Infect. Dis. 42:244–251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases