Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins - PubMed (original) (raw)
Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins
Rajesh Ghai et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Transit of proteins through the endosomal organelle following endocytosis is critical for regulating the homeostasis of cell-surface proteins and controlling signal transduction pathways. However, the mechanisms that control these membrane-transport processes are poorly understood. The Phox-homology (PX) domain-containing proteins sorting nexin (SNX) 17, SNX27, and SNX31 have emerged recently as key regulators of endosomal recycling and bind conserved Asn-Pro-Xaa-Tyr-sorting signals in transmembrane cargos via an atypical band, 4.1/ezrin/radixin/moesin (FERM) domain. Here we present the crystal structure of the SNX17 FERM domain bound to the sorting motif of the P-selectin adhesion protein, revealing both the architecture of the atypical FERM domain and the molecular basis for recognition of these essential sorting sequences. We further show that the PX-FERM proteins share a promiscuous ability to bind a wide array of putative cargo molecules, including receptor tyrosine kinases, and propose a model for their coordinated molecular interactions with membrane, cargo, and regulatory proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Structure of the atypical SNX17 FERM domain. (A) Ribbon representation of the atypical SNX17 FERM domain crystal structure depicting the three submodules F1, F2, and F3 in red, green, and blue, respectively. (B) Overlay of the FERM domain of SNX17 with radixin [Protein Data Bank (PDB) ID, 1GC7)] (28). Brighter worms show SNX17; paler colors indicate radixin. The α3B helix, present in all other FERM domains but absent in SNX17, SNX27, and SNX31, is indicated by thicker green worms. The cloverleaf arrangement of the three submodules of SNX17 shows overall similarity to radixin but differs dramatically from the extended conformation of the talin protein (27). (C and D) Solution SAXS data are shown for SNX17 (C) and SNX27 (D) FERM domains. Both SNX17 and SNX27 FERM domains adopt compact, globular structures in solution, consistent with the SNX17 FERM domain crystal structure. (Left) Experimental SAXS profiles in black overlaid with the theoretical profiles calculated from the SNX17 crystal structure by CRYSOL (green) or the ab initio model determined by the program GASBOR (red). Insets show the Guinier plots for the experimental data. (Center) P(r) functions derived from the SAXS data. (Right) Averaged (gray) and filtered (blue) structural envelopes from GASBOR. The crystal structure of the SNX17 FERM domain is shown in ribbon representation and was docked into the ab initio envelopes using SUPCOMB20.
Fig. 2.
Structure of the SNX17 FERM domain in complex with the P-selectin ICD. (A) Table of NPxY/NxxY sequences from cargo ICDs previously found to interact with SNX17. Surrounding sequences from the ICDs are shown. See text for references. (B) The crystal structure of the SNX17 FERM domain bound to the P-selectin ICD shows that the NPxY/NxxY motif binds the F3 submodule of SNX17. The P-selectin peptide is shown in yellow stick representation, and refined 2_F_o-_F_c electron density contoured at 1.5σ for the bound peptide is shown in magenta. (C) Surface representation of the P-selectin ICD-binding groove formed by the SNX17 helix α1C (blue) and strand β5C (magenta). The peptide residues that form critical contacts with the β5C-α1C groove are indicated by dashed circles. (D) Detailed view of the interaction between the F3 lobe of SNX17 and the ICD of P-selectin. The P-selectin peptide is shown in explicit atomic model (yellow cylinders), and the F3 module is represented as ribbons. The amino acids of the SNX17 F3 domain that are involved in the interaction with the P-selectin ICD are shown as blue cylinders, and hydrogen bonds are depicted by dashed lines. Interfacial water molecules are shown as gray spheres. (E) Mapping residues that are conserved among SNX17, SNX27, and SNX31 PX-FERM proteins onto the SNX17 structure highlights the conservation of the peptide-binding site. The surface of the SNX17 FERM domain colored white (nonconserved) to green (highly conserved). The dashed ellipse indicates the peptide-binding groove.
Fig. 3.
Comparison of the SNX17/P-selectin complex with distantly related endocytic and signaling complexes. (A) The SNX17 F3 module is shown in blue bound to P-selectin ICD, shown in yellow, and the critical N−3 and Y0 of the NPxY/NxxY motif are highlighted in surface representation. The structure is similar to several other endocytic and signaling complexes that are shown for comparison: the PTB domain of FE65 bound to the APP ICD (PDB ID, 3DXC) (36, 56), the F3 subdomain of talin bound to the β1 integrin ICD (PDB ID, 3G9W), (37), and the PTB domain of ARH bound to the LRP1 ICD (PDB ID, 3SO6) (5). In each case, N−3 and Y0 side chains from the NPxY/NxxY motif are represented in transparent green surface, and the N and C termini of the bound ICDs are indicated. (B) Detailed comparison of key side chains from the ICD of APP, β1 integrin, and LRP1 with respect to the SNX17 FERM domain/P-selectin complex demonstrates their similar relative orientations. All peptide side chains are colored as in A. The interacting side chains of SNX17 are shown as blue cylinders. (C) The P-selectin ICD contains overlapping Yxxϕ and NPxY/NxxY sorting motifs. The structure of the P-selectin ICD bound to SNX17 (yellow) is overlaid with the contiguous P-selectin YGVF-containing peptide bound to the AP2 μ2 subunit (magenta) (38).
Fig. 4.
Mutational analysis of the NPxY/NxxY cargo-binding site of PX-FERM proteins. Mutagenesis of the NPxY/NxxY-binding site of SNX17 and the analogous region of SNX27 inhibits peptide motif interactions. A full-sequence alignment of the proteins is shown in
Fig. S2
. (A) SNX17 was tested for its interaction with the GST-P-selectin ICD in GST pull-down assays. The upper panel shows the Coomassie-stained gel of the pull-down samples; the lower panel shows a Western blot using anti-His antibody to detect SNX17. (B) Binding of SNX17 to NPxY/NxxY motif-containing peptides derived from the ICD of P-selectin (Left) and APP (Right) was examined by ITC, using 35 μM protein and 375 μM P-selectin peptide (GTYGVFT
NAAY
DP) or 1 mM APP peptide (CKQNGYE
NPTY
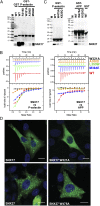
KFFE). Upper panels show raw data; and lower panels show integrated normalized data. (C) SNX27ΔPDZ was tested for its interaction with GST-APP and GST-P-selectin ICD sequences in GST pull-down assays. The upper panel shows the Coomassie-stained gel of the pull-down samples, and the lower panel shows a Western blot using anti-His antibody to detect SNX27ΔPDZ. Mutations analogous to the SNX17 motif-binding site also abolish interaction of SNX27 with the ICD sequences. (D) SNX17 and SNX27 were transiently expressed in CHO-InsR cells and detected by immunofluorescence labeling of the C-terminal myc tags (green). DAPI nuclear labeling is shown in blue. Both wild-type proteins show punctate endosomal recruitment, as does the SNX27 protein with an altered NPxY/NxxY-binding site (W475A mutant). The SNX17-binding mutant, however, shows a diffuse cytoplasmic distribution. (Scale bars, 10 μm.)
Fig. 5.
PX-FERM proteins show promiscuous binding to many putative NPxY/NxxY motif-containing cargos. To examine the potential of PX-FERM proteins to interact with other receptors containing NPxY/NxxY, a peptide array screen was performed based on the array developed by Smith et al. (42). The arrays comprise 126 peptides derived from transmembrane proteins from the human proteome that contain NPxY/NxxY sequences and are probed in both their phosphorylated and nonphosphorylated Y0 states. Cargo proteins from similar classes are highlighted. The specific constructs tested were GST-SNX17, GST-SNX31, and GST-SNX27ΔPDZ. GST alone was the negative control.
Fig. 6.
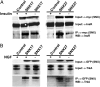
Interaction of PX-FERM proteins with RTKs in cells. SNX17 and SNX27 with either myc or GFP tags were transiently expressed with different RTKs and tested for binding to InsR in CHO cells stably expressing the InsR (A) or to TrkA in transiently expressing HEK293 cells (B). Proteins were immunoprecipitated with indicated antibodies before and after stimulation with respective agonists, insulin (100 nM for 15 min), or NGF (3 nM for 10 min). Control experiments were performed in the absence of either SNX17 or SNX27. For TrkA experiments, proteins were crosslinked with dithiobis(succinimidylpropionate) before immunoprecipitation.
Fig. 7.
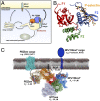
A model for membrane-coincident binding of PX-FERM proteins to cargo, lipids, and regulatory proteins. The model shows the interaction of PX-FERM proteins with endosomal cargo (both PDZbm and NPxY/NxxY-containing molecules), the endosomal lipid PI3P, and a putative small GTPase ligand. The model is derived from the low-resolution structure of the SNX27 protein determined by SAXS (
Fig. S5
), overlaid with high-resolution structures of the different ligand-bound domains determined by X-ray crystallography (this study and refs. , , and 44).
Fig. P1.
Structure and function of the PX-FERM proteins. (A) The PX-FERM proteins regulate endosomal trafficking and recycling of cargo molecules containing NPxY/NxxY sorting motifs. (B) The crystal structure of the SNX17 FERM domain in complex with the sorting motif from P-selectin (yellow) reveals how the peptide binds the F3 module (blue) within a conserved complementary groove. (C) Based on this structure and on previous studies, we propose a model for the coordinated interaction of PX-FERM proteins with cargo molecules, membrane lipids, and regulatory proteins, as required for their function at the endosomal membrane.
Similar articles
- Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases.
Ghai R, Mobli M, Norwood SJ, Bugarcic A, Teasdale RD, King GF, Collins BM. Ghai R, et al. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7763-8. doi: 10.1073/pnas.1017110108. Epub 2011 Apr 21. Proc Natl Acad Sci U S A. 2011. PMID: 21512128 Free PMC article. - Measuring interactions of FERM domain-containing sorting Nexin proteins with endosomal lipids and cargo molecules.
Ghai R, Mobli M, Collins BM. Ghai R, et al. Methods Enzymol. 2014;534:331-49. doi: 10.1016/B978-0-12-397926-1.00019-6. Methods Enzymol. 2014. PMID: 24359963 - SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27.
Yong X, Zhao L, Hu W, Sun Q, Ham H, Liu Z, Ren J, Zhang Z, Zhou Y, Yang Q, Mo X, Hu J, Billadeau DD, Jia D. Yong X, et al. Proc Natl Acad Sci U S A. 2021 Sep 7;118(36):e2105510118. doi: 10.1073/pnas.2105510118. Proc Natl Acad Sci U S A. 2021. PMID: 34462354 Free PMC article. - Recognising the signals for endosomal trafficking.
Weeratunga S, Paul B, Collins BM. Weeratunga S, et al. Curr Opin Cell Biol. 2020 Aug;65:17-27. doi: 10.1016/j.ceb.2020.02.005. Epub 2020 Mar 7. Curr Opin Cell Biol. 2020. PMID: 32155566 Review. - Towards a molecular understanding of endosomal trafficking by Retromer and Retriever.
Chen KE, Healy MD, Collins BM. Chen KE, et al. Traffic. 2019 Jul;20(7):465-478. doi: 10.1111/tra.12649. Traffic. 2019. PMID: 30993794 Review.
Cited by
- OCRL1 Deficiency Affects the Intracellular Traffic of ApoER2 and Impairs Reelin-Induced Responses.
Fuentealba LM, Pizarro H, Marzolo MP. Fuentealba LM, et al. Biomolecules. 2024 Jul 5;14(7):799. doi: 10.3390/biom14070799. Biomolecules. 2024. PMID: 39062513 Free PMC article. - Structural basis of arc binding to synaptic proteins: implications for cognitive disease.
Zhang W, Wu J, Ward MD, Yang S, Chuang YA, Xiao M, Li R, Leahy DJ, Worley PF. Zhang W, et al. Neuron. 2015 Apr 22;86(2):490-500. doi: 10.1016/j.neuron.2015.03.030. Epub 2015 Apr 9. Neuron. 2015. PMID: 25864631 Free PMC article. - Myosin MyTH4-FERM structures highlight important principles of convergent evolution.
Planelles-Herrero VJ, Blanc F, Sirigu S, Sirkia H, Clause J, Sourigues Y, Johnsrud DO, Amigues B, Cecchini M, Gilbert SP, Houdusse A, Titus MA. Planelles-Herrero VJ, et al. Proc Natl Acad Sci U S A. 2016 May 24;113(21):E2906-15. doi: 10.1073/pnas.1600736113. Epub 2016 May 10. Proc Natl Acad Sci U S A. 2016. PMID: 27166421 Free PMC article. - SNX27-Retromer directly binds ESCPE-1 to transfer cargo proteins during endosomal recycling.
Simonetti B, Guo Q, Giménez-Andrés M, Chen KE, Moody ERR, Evans AJ, Chandra M, Danson CM, Williams TA, Collins BM, Cullen PJ. Simonetti B, et al. PLoS Biol. 2022 Apr 13;20(4):e3001601. doi: 10.1371/journal.pbio.3001601. eCollection 2022 Apr. PLoS Biol. 2022. PMID: 35417450 Free PMC article. - The Retromer Complex and Sorting Nexins in Neurodegenerative Diseases.
Zhang H, Huang T, Hong Y, Yang W, Zhang X, Luo H, Xu H, Wang X. Zhang H, et al. Front Aging Neurosci. 2018 Mar 26;10:79. doi: 10.3389/fnagi.2018.00079. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29632483 Free PMC article. Review.
References
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
- Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265(6):3116–3123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous