Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer - PubMed (original) (raw)
Comparative Study
doi: 10.1136/jclinpath-2012-201240. Epub 2013 Feb 5.
Barbara Angulo, Belen Gomez, Debbie Mair, Rebeca Martinez, Esther Conde, Felice Shieh, Julie Tsai, Jeffrey Vaks, Robert Current, H Jeffrey Lawrence, David Gonzalez de Castro
Affiliations
- PMID: 23386666
- PMCID: PMC3632986
- DOI: 10.1136/jclinpath-2012-201240
Free PMC article
Comparative Study
Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer
Fernando Lopez-Rios et al. J Clin Pathol. 2013 May.
Free PMC article
Abstract
Aim: To conduct a methods correlation study of three different assays for the detection of mutations at EGFR gene in human formalin-fixed paraffin-embedded tumour (FFPET) specimens of non-small cell lung carcinomas (NSCLC).
Methods: We conducted a 2-site method comparison study of two european conformity (CE) in vitro diagnostic (IVD)-marked assays, the cobas EGFR Mutation Test and the Therascreen EGFR29 Mutation Kit, and 2× bidirectional Sanger sequencing. We blind-tested 124 NSCLC FFPET specimens with all three methods; the cobas test was performed at both sites. Positive (PPA) and negative percent agreements (NPA) were determined for the cobas test versus each of the other two methods. Specimens yielding discordant test results between methods were further tested using quantitative massively parallel pyrosequencing (MPP).
Results: PPA between cobas and Sanger was 98.8%; NPA was 79.3%. Overall there were seven discordant results. MPP confirmed an exon 19 deletion in two cases and L858R mutation in four cases. PPA between cobas and Therascreen was 98.9% and NPA was 100%. There was one discordant result. Reproducibility of the cobas test between the two sites was 99.2%.
Conclusions: The invalid rates for the cobas test and Therascreen were lower than Sanger sequencing. The cobas and Therascreen assays showed a high degree of concordance, and both were more sensitive for the detection of exon 19 deletion and L858R mutations than Sanger. The cobas test was highly reproducible between the two testing sites, used the least amount of DNA input and was the only test with automated results reporting.
Figures
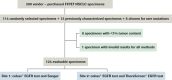
Figure 1
Study design and specimen selection. EGFR, epidermal growth factor receptor; FFPET, formalin-fixed paraffin-embedded tissue; NSCLC, non-small cell lung cancer.
Similar articles
- Analytic performance studies and clinical reproducibility of a real-time PCR assay for the detection of epidermal growth factor receptor gene mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer.
O'Donnell P, Ferguson J, Shyu J, Current R, Rehage T, Tsai J, Christensen M, Tran HB, Chien SS, Shieh F, Wei W, Lawrence HJ, Wu L, Schilling R, Bloom K, Maltzman W, Anderson S, Soviero S. O'Donnell P, et al. BMC Cancer. 2013 Apr 27;13:210. doi: 10.1186/1471-2407-13-210. BMC Cancer. 2013. PMID: 23621958 Free PMC article. - Analytical performance of the cobas EGFR mutation assay for Japanese non-small-cell lung cancer.
Kimura H, Ohira T, Uchida O, Matsubayashi J, Shimizu S, Nagao T, Ikeda N, Nishio K. Kimura H, et al. Lung Cancer. 2014 Mar;83(3):329-33. doi: 10.1016/j.lungcan.2013.12.012. Epub 2014 Jan 3. Lung Cancer. 2014. PMID: 24439568 - A comparison of three methods for detecting KRAS mutations in formalin-fixed colorectal cancer specimens.
Gonzalez de Castro D, Angulo B, Gomez B, Mair D, Martinez R, Suarez-Gauthier A, Shieh F, Velez M, Brophy VH, Lawrence HJ, Lopez-Rios F. Gonzalez de Castro D, et al. Br J Cancer. 2012 Jul 10;107(2):345-51. doi: 10.1038/bjc.2012.259. Epub 2012 Jun 19. Br J Cancer. 2012. PMID: 22713664 Free PMC article. - Multisite analytic performance studies of a real-time polymerase chain reaction assay for the detection of BRAF V600E mutations in formalin-fixed, paraffin-embedded tissue specimens of malignant melanoma.
Anderson S, Bloom KJ, Vallera DU, Rueschoff J, Meldrum C, Schilling R, Kovach B, Lee JR, Ochoa P, Langland R, Halait H, Lawrence HJ, Dugan MC. Anderson S, et al. Arch Pathol Lab Med. 2012 Nov;136(11):1385-91. doi: 10.5858/arpa.2011-0505-OA. Epub 2012 Feb 14. Arch Pathol Lab Med. 2012. PMID: 22332713 Clinical Trial. - Profile of the therascreen® EGFR RGQ PCR kit as a companion diagnostic for gefitinib in non-small cell lung cancer.
Hsiue EH, Lee JH, Lin CC, Yang JC. Hsiue EH, et al. Expert Rev Mol Diagn. 2016 Dec;16(12):1251-1257. doi: 10.1080/14737159.2016.1248414. Epub 2016 Oct 27. Expert Rev Mol Diagn. 2016. PMID: 27737606 Review.
Cited by
- Real-time PCR and targeted next-generation sequencing in the detection of low level EGFR mutations: Instructive case analyses.
Cheng YW, Stefaniuk C, Jakubowski MA. Cheng YW, et al. Respir Med Case Rep. 2019 Jul 10;28:100901. doi: 10.1016/j.rmcr.2019.100901. eCollection 2019. Respir Med Case Rep. 2019. PMID: 31367517 Free PMC article. - Cost-effectiveness analysis comparing companion diagnostic tests for EGFR, ALK, and ROS1 versus next-generation sequencing (NGS) in advanced adenocarcinoma lung cancer patients.
Schluckebier L, Caetano R, Garay OU, Montenegro GT, Custodio M, Aran V, Gil Ferreira C. Schluckebier L, et al. BMC Cancer. 2020 Sep 14;20(1):875. doi: 10.1186/s12885-020-07240-2. BMC Cancer. 2020. PMID: 32928143 Free PMC article. - Tissue and Plasma-Based Highly Sensitive Blocker Displacement Amplicon Nanopore Sequencing for EGFR Mutations in Lung Cancer.
Akkhasutthikun P, Kaewsapsak P, Nimsamer P, Klomkliew P, Visedthorn S, Chanchaem P, Teerapakpinyo C, Payungporn S, Luangdilok S. Akkhasutthikun P, et al. Cancer Res Treat. 2024 Apr;56(2):455-463. doi: 10.4143/crt.2023.1108. Epub 2023 Nov 20. Cancer Res Treat. 2024. PMID: 37986562 Free PMC article. - Lung adenocarcinoma in a patient with a cis EGFR L858R-K860I doublet mutation identified using NGS-based profiling test: Negative diagnosis on initial companion test and successful treatment with osimertinib.
Onozawa H, Saito H, Sunami K, Kubo T, Yamamoto N, Kasajima R, Ohtsu T, Hiroshima Y, Kanamori H, Yokose T, Miyagi Y. Onozawa H, et al. Thorac Cancer. 2020 Dec;11(12):3599-3604. doi: 10.1111/1759-7714.13694. Epub 2020 Oct 9. Thorac Cancer. 2020. PMID: 33034420 Free PMC article. - Comparison of detection methods of EGFR T790M mutations using plasma, serum, and tumor tissue in EGFR-TKI-resistant non-small cell lung cancer.
Kobayashi K, Naoki K, Manabe T, Masuzawa K, Hasegawa H, Yasuda H, Kawada I, Soejima K, Betsuyaku T. Kobayashi K, et al. Onco Targets Ther. 2018 Jun 6;11:3335-3343. doi: 10.2147/OTT.S161745. eCollection 2018. Onco Targets Ther. 2018. PMID: 29922072 Free PMC article.
References
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42 - PubMed
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57 - PubMed
- Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trial. J Clin Oncol 2010;28:744–52 - PubMed
- Agency EM. Iressa (gefitinib)—EPAR summary for the public 2009.
- Genentech IaAPU, Inc. Tarceva Prescribing Information 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous