The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction - PubMed (original) (raw)
The CUL3-KLHL3 E3 ligase complex mutated in Gordon's hypertension syndrome interacts with and ubiquitylates WNK isoforms: disease-causing mutations in KLHL3 and WNK4 disrupt interaction
Akihito Ohta et al. Biochem J. 2013.
Abstract
The WNK (with no lysine kinase)-SPAK (SPS1-related proline/alanine-rich kinase)/OSR1 (oxidative stress-responsive kinase 1) signalling pathway plays an important role in controlling mammalian blood pressure by modulating the activity of ion co-transporters in the kidney. Recent studies have identified Gordon's hypertension syndrome patients with mutations in either CUL3 (Cullin-3) or the BTB protein KLHL3 (Kelch-like 3). CUL3 assembles with BTB proteins to form Cullin-RING E3 ubiquitin ligase complexes. To explore how a CUL3-KLHL3 complex might operate, we immunoprecipitated KLHL3 and found that it associated strongly with WNK isoforms and CUL3, but not with other components of the pathway [SPAK/OSR1 or NCC (Na(+)/Cl(-) co-transporter)/NKCC1 (Na(+)/K(+)/2Cl(-) co-transporter 1)]. Strikingly, 13 out of the 15 dominant KLHL3 disease mutations analysed inhibited binding to WNK1 or CUL3. The recombinant wild-type CUL3-KLHL3 E3 ligase complex, but not a disease-causing CUL3-KLHL3[R528H] mutant complex, ubiquitylated WNK1 in vitro. Moreover, siRNA (small interfering RNA)-mediated knockdown of CUL3 increased WNK1 protein levels and kinase activity in HeLa cells. We mapped the KLHL3 interaction site in WNK1 to a non-catalytic region (residues 479-667). Interestingly, the equivalent region in WNK4 encompasses residues that are mutated in Gordon's syndrome patients. Strikingly, we found that the Gordon's disease-causing WNK4[E562K] and WNK4[Q565E] mutations, as well as the equivalent mutation in the WNK1[479-667] fragment, abolished the ability to interact with KLHL3. These results suggest that the CUL3-KLHL3 E3 ligase complex regulates blood pressure via its ability to interact with and ubiquitylate WNK isoforms. The findings of the present study also emphasize that the missense mutations in WNK4 that cause Gordon's syndrome strongly inhibit interaction with KLHL3. This could elevate blood pressure by increasing the expression of WNK4 thereby stimulating inappropriate salt retention in the kidney by promoting activation of the NCC/NKCC2 ion co-transporters. The present study reveals how mutations that disrupt the ability of an E3 ligase to interact with and ubiquitylate a critical cellular substrate such as WNK isoforms can trigger a chronic disease such as hypertension.
Figures
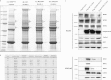
Figure 1. Evidence that KLHL3 associates with WNK isoforms and interaction is impaired by Gordon's syndrome mutations
(A) Control HEK-293 cells or HEK-293 cells stably expressing the indicated forms of wild-type and mutant KLHL3 possessing an N-terminal FLAG tag were cultured in the presence of 1 μg/ml tetracyclin to induce expression of KLHL3. Cells were lysed and KLHL3 was immunoprecipitated from 5 mg of extract using an anti-FLAG antibody covalently coupled to agarose. The immunoprecipitates were electrophoresed on an SDS polyacrylamide gel and protein bands visualized following Colloidal Blue staining. Molecular masses are shown on the left-hand side in kDa. (B) The labelled Colloidal Blue-stained bands identified in (A) were excised from the gel and digested with trypsin, and their identities were determined by tryptic peptide mass-spectral fingerprinting, as described in the Materials and methods section. Accession numbers are for the NCBI Entrez Protein database. Mascot protein scores >67 were considered significant (P<0.05). N.P.D., no significant protein identity determined. MW, molecular mass. (C) As (A), except that the immunoprecipitates were immunoblotted with the indicated antibodies. Similar results were obtained in three separate experiments. Molecular masses are shown on the left-hand side in kDa.
Figure 2. Evidence that dominant KLHL3 Gordon's syndrome mutations impair binding to CUL3 or WNK
(A) Schematic representation of the domain structure of KLHL3 with the positions of the dominant Gordon's syndrome-associated mutations illustrated. The amino acid boundaries of the domains are indicated. PHAII, pseudohypoaldosteronism type II. (B) Control HEK-293 cells or HEK-293 cells stably expressing the indicated forms of wild-type (WT) and mutant KLHL3 possessing an N-terminal FLAG tag were cultured in the presence of 1 μg/ml tetracyclin to induce expression of KLHL3. Cells were lysed and KLHL3 was immunoprecipitated (IP) from 0.2 mg of extract using an anti-FLAG antibody covalently coupled to agarose. The immunoprecipitates (upper panel) as well as whole-cell extracts (lysates, lower panel) were immunoblotted with the indicated antibodies. All gels were immunoblotted in parallel. The broken lines indicate that samples were run on separate gels. Similar results were obtained in three separate experiments. Molecular masses are shown on the left-hand side in kDa.
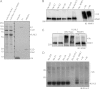
Figure 3. Evidence that wild-type or dominant KLHL3 Gordon's syndrome mutations do not interact with SPAK/OSR1 or NKCC1 or NCC
(A) HEK-293 stably expressing the wild-type (WT) and indicated mutants of KLHL3 possessing an N-terminal FLAG epitope were cultured in the presence of 1 μg/ml tetracyclin to induce the expression of KLHL3. WNK1, SPAK/OSR1 and NKCC1 were separately immunoprecipitated (IP) from 0.2 mg of extract using previously characterized immunoprecipitating antibodies covalently coupled to Protein G–Sepharose. Whole-cell extracts (lysates, upper panel) as well as immunoprecipitates (lower panels) were immunoblotted with the indicated antibodies. (B) N-terminal GFP-tagged wild-type KLHL3 was transiently transfected into previously characterized HEK-293 cells stably expressing FLAG–NCC [4]. At 24 h post-transfection cells were cultured for a further 24 h with 1 μg/ml tetracyclin to induce NCC expression before lysis. GFP–KLHL3 was immunoprecipitated from 0.2 mg of extract employing anti-GFP antibody covalently coupled to agarose. Whole-cell extracts (lysates, left-hand panel) as well as immunoprecipitates (right-hand panel) were immunoblotted with the indicated antibodies. Similar results were obtained in three separate experiments. Molecular masses are shown on the left-hand side in kDa.
Figure 4. WNK1 is a substrate for the CUL3–KLHL3 complex in vitro
(A) Coomassie Blue-stained SDS/PAGE gel of Cul3–Rbx1–KLHL3 wild-type, Cul3–Rbx1–KHLH3[R528H], ubiquitin (Ub), E1 and UBE2D3 used in the_in vitro_ reactions in which all tags used for affinity purification of each protein has been removed with the rTEV protease and the proteins were re-purified. The line indicates that samples were run on separate gels. (B) Immunoprecipitated WNK1 from HEK-293 cells was incubated at 30°C for 30 min with purified E1, E2 (UBE2D3), Cullin3–Rbx1, KLHL3 and ubiquitin in the presence of 1 mM ATP. Control reactions were performed whereby one of the proteins required for ubiquitylation was omitted from the reaction as indicated. Samples were subjected to immunoblot (IB) analysis with an anti-WNK1 antibody. (C) Ubiquitylation reactions were performed as in (B) and the ability of KLHL3 R528H to promote WNK1 ubiquitylation was analysed. Independent duplicate reactions are shown. (D) Ubiquitylation reactions were performed as in (B) and the ubiquitylation of KLHL3 was analysed by immunoblot detection with anti-KLHL3 total antibody. Similar results were obtained in three separate experiments. Molecular masses are shown on the left-hand side in kDa.
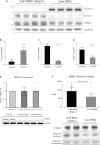
Figure 5. Evidence that CUL3 knockdown enhances WNK1 expression and activity
HeLa cells were treated with 50 nM scrambled siRNA or CUL3-directed siRNA (probe 1) for 5 days and cells were lysed. (A) Cell lysates were subjected to immunoblotting with the indicated antibody. Each lane contains cell extract from an independent dish of cells from an identical experiment undertaken on the same day. (B–D) Quantitative LI-COR immunoblot analysis was undertaken and the ratio of CUL3 (B), KLHL3 (C) and WNK1 (D) to GAPDH was quantified. Results are means±S.E.M. for two independent samples each assayed in duplicates with P values indicated. (E) Total mRNA was isolated from cells and WNK1 mRNA levels were determined by qRT-PCR. Data were normalized to internal GAPDH and RPL13A controls as described in the Materials and methods section. Results are means±S.D. for three independent samples each assayed in triplicate. (F) WNK1 was immunoprecipitated from 0.25 mg of cell extracts and assayed for its ability to phosphorylate kinase inactive SPAK expressed in E. coli cells. The top panel displays activity in 32P radioactivity incorporated into kinase inactive SPAK (c.p.m.) as mean±S.D. The lower panels display representative immunoblot analysis of WNK1, autorad of phosphorylated SPAK and Coomassie Blue staining of kinase-inactive SPAK employed in the kinase assay. Similar findings were made with an independent CUL3-directed siRNA (probe 2); see data in Supplementary Figure S1 (at
http://www.biochemj.org/bj/451/bj4510111add.htm
). Cont, control; KD, kinase-dead; IP, immunoprecipitation; RNAi, RNA interference.
Figure 6. Evidence that WNK4 Gordon's syndrome missense mutations impair binding to KLHL3
(A) Schematic diagram of WNK1 and WNK4 isoform structures and the relative placement of their kinase domain, auto-inhibitory domain (AID) and predicted coiled-coiled domains (CCD). Also highlighted in WNK4 is the location of the E562K and Q565E mutations found in Gordon's hypertension patients. CCD, coiled-coil; CT, C-terminal. (B) HEK-293 cells stably expressing GFP–KLHL3 were transfected with GST-tagged constructs encoding the indicated fragments of WNK1. At 24 h post-transfection the cells were treated with tetracyclin (1 μg/ml) to induce KLHL3 expression and cells were lysed after a further 24 h. Cell extracts were subjected to affinity purification on glutathione–Sepharose and immunoblotted with an anti-GFP antibody (top panel) or subjected to affinity purification with an anti-GFP antibody and immunoblotted with an anti-GST antibody (upper middle panel). Cell lysates were also subjected to immunoblot analysis with an anti-GST (lower middle panel) or anti-GFP (bottom panel) antibody. Similar results were obtained in two independent experiments. (C) HEK-293 cells stably expressing the wild-type (WT) and indicated mutant forms of FLAG-tagged WNK4 were transfected with wild-type GFP-tagged KLHL3. At 24 h post-transfection cells were treated with tetracyclin (1 μg/ml) to induce WNK4 expression and cells were lysed after 24 h. Extracts were subjected to affinity purification using an anti-FLAG antibody (to immunoprecipitate WNK4; upper panels) or an anti-GFP antibody (to immunoprecipitate KLHL3; lower panels) and immunoblotted with the indicated antibodies. Cell lysates were also immunoblotted with the indicated antibodies. Similar results were obtained in two independent experiments. (D) As (B) except that the wild-type and indicated mutants of the WNK1[479–667] fragment were analysed for their ability to interact with KLHL3. IP, immunoprecipitation. Molecular masses are shown on the left-hand side of the gels in kDa.
Similar articles
- Structural and biochemical characterization of the KLHL3-WNK kinase interaction important in blood pressure regulation.
Schumacher FR, Sorrell FJ, Alessi DR, Bullock AN, Kurz T. Schumacher FR, et al. Biochem J. 2014 Jun 1;460(2):237-46. doi: 10.1042/BJ20140153. Biochem J. 2014. PMID: 24641320 Free PMC article. - Kelch-like 3/Cullin 3 ubiquitin ligase complex and WNK signaling in salt-sensitive hypertension and electrolyte disorder.
Sohara E, Uchida S. Sohara E, et al. Nephrol Dial Transplant. 2016 Sep;31(9):1417-24. doi: 10.1093/ndt/gfv259. Epub 2015 Jul 6. Nephrol Dial Transplant. 2016. PMID: 26152401 Review. - Kelch-like 3 and Cullin 3 regulate electrolyte homeostasis via ubiquitination and degradation of WNK4.
Shibata S, Zhang J, Puthumana J, Stone KL, Lifton RP. Shibata S, et al. Proc Natl Acad Sci U S A. 2013 May 7;110(19):7838-43. doi: 10.1073/pnas.1304592110. Epub 2013 Apr 1. Proc Natl Acad Sci U S A. 2013. PMID: 23576762 Free PMC article. - Role of KLHL3 and dietary K+ in regulating KS-WNK1 expression.
Ostrosky-Frid M, Chávez-Canales M, Zhang J, Andrukhova O, Argaiz ER, Lerdo-de-Tejada F, Murillo-de-Ozores A, Sanchez-Navarro A, Rojas-Vega L, Bobadilla NA, Vazquez N, Castañeda-Bueno M, Alessi DR, Gamba G. Ostrosky-Frid M, et al. Am J Physiol Renal Physiol. 2021 May 1;320(5):F734-F747. doi: 10.1152/ajprenal.00575.2020. Epub 2021 Mar 8. Am J Physiol Renal Physiol. 2021. PMID: 33682442 Free PMC article. - WNK-SPAK/OSR1-NCC kinase signaling pathway as a novel target for the treatment of salt-sensitive hypertension.
Brown A, Meor Azlan NF, Wu Z, Zhang J. Brown A, et al. Acta Pharmacol Sin. 2021 Apr;42(4):508-517. doi: 10.1038/s41401-020-0474-7. Epub 2020 Jul 28. Acta Pharmacol Sin. 2021. PMID: 32724175 Free PMC article. Review.
Cited by
- Alternatively spliced proline-rich cassettes link WNK1 to aldosterone action.
Roy A, Al-Qusairi L, Donnelly BF, Ronzaud C, Marciszyn AL, Gong F, Chang YP, Butterworth MB, Pastor-Soler NM, Hallows KR, Staub O, Subramanya AR. Roy A, et al. J Clin Invest. 2015 Sep;125(9):3433-48. doi: 10.1172/JCI75245. Epub 2015 Aug 4. J Clin Invest. 2015. PMID: 26241057 Free PMC article. - Distal convoluted tubule.
McCormick JA, Ellison DH. McCormick JA, et al. Compr Physiol. 2015 Jan;5(1):45-98. doi: 10.1002/cphy.c140002. Compr Physiol. 2015. PMID: 25589264 Free PMC article. Review. - Role of the Ubiquitin Proteasome System in the Regulation of Blood Pressure: A Review.
Yamazaki O, Hirohama D, Ishizawa K, Shibata S. Yamazaki O, et al. Int J Mol Sci. 2020 Jul 28;21(15):5358. doi: 10.3390/ijms21155358. Int J Mol Sci. 2020. PMID: 32731518 Free PMC article. Review. - Hypertension-causing cullin 3 mutations disrupt COP9 signalosome binding.
Cornelius RJ, Yang CL, Ellison DH. Cornelius RJ, et al. Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F204-F208. doi: 10.1152/ajprenal.00497.2019. Epub 2019 Dec 9. Am J Physiol Renal Physiol. 2020. PMID: 31813255 Free PMC article. Review. - COP9 signalosome deletion promotes renal injury and distal convoluted tubule remodeling.
Cornelius RJ, Nelson JW, Su XT, Yang CL, Ellison DH. Cornelius RJ, et al. Am J Physiol Renal Physiol. 2022 Jul 1;323(1):F4-F19. doi: 10.1152/ajprenal.00436.2021. Epub 2022 May 9. Am J Physiol Renal Physiol. 2022. PMID: 35532068 Free PMC article.
References
- Wilson F. H., Disse-Nicodeme S., Choate K. A., Ishikawa K., Nelson-Williams C., Desitter I., Gunel M., Milford D. V., Lipkin G. W., Achard J. M., et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
- Delpire E., Gagnon K. B. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem. J. 2008;409:321–331. - PubMed
- Richardson C., Rafiqi F. H., Karlsson H. K., Moleleki N., Vandewalle A., Campbell D. G., Morrice N. A., Alessi D. R. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J. Cell Sci. 2008;121:675–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 243019/ERC_/European Research Council/International
- MC_U127070193/MRC_/Medical Research Council/United Kingdom
- MC_UU_12016/2/MRC_/Medical Research Council/United Kingdom
- MC_UU_12016/7/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases