Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort - PubMed (original) (raw)
Paternal obesity is associated with IGF2 hypomethylation in newborns: results from a Newborn Epigenetics Study (NEST) cohort
Adelheid Soubry et al. BMC Med. 2013.
Abstract
Background: Data from epidemiological and animal model studies suggest that nutrition during pregnancy may affect the health status of subsequent generations. These transgenerational effects are now being explained by disruptions at the level of the epigenetic machinery. Besides in vitro environmental exposures, the possible impact on the reprogramming of methylation profiles at imprinted genes at a much earlier time point, such as during spermatogenesis or oogenesis, has not previously been considered. In this study, our aim was to determine associations between preconceptional obesity and DNA methylation profiles in the offspring, particularly at the differentially methylated regions (DMRs) of the imprinted Insulin-like Growth Factor 2 (IGF2) gene.
Methods: We examined DNA from umbilical cord blood leukocytes from 79 newborns, born between July 2005 and November 2006 at Duke University Hospital, Durham, NC. Their mothers participated in the Newborn Epigenetics Study (NEST) during pregnancy. Parental characteristics were obtained via standardized questionnaires and medical records. DNA methylation patterns at two DMRs were analyzed by bisulfite pyrosequencing; one DMR upstream of IGF2 (IGF2 DMR), and one DMR upstream of the neighboring H19 gene (H19 DMR). Multiple regression models were used to determine potential associations between the offspring's DNA methylation patterns and parental obesity before conception. Obesity was defined as body mass index (BMI) ≥30 kg/m².
Results: Hypomethylation at the IGF2 DMR was associated with paternal obesity. Even after adjusting for several maternal and newborn characteristics, we observed a persistent inverse association between DNA methylation in the offspring and paternal obesity (β-coefficient was -5.28, P = 0.003). At the H19 DMR, no significant associations were detected between methylation patterns and paternal obesity. Our data suggest an increase in DNA methylation at the IGF2 and H19 DMRs among newborns from obese mothers, but a larger study is warranted to further explore the potential effects of maternal obesity or lifestyle on the offspring's epigenome.
Conclusions: While our small sample size is limited, our data indicate a preconceptional impact of paternal obesity on the reprogramming of imprint marks during spermatogenesis. Given the biological importance of imprinting fidelity, our study provides evidence for transgenerational effects of paternal obesity that may influence the offspring's future health status.
Figures
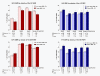
Figure 1
Methylation at the IGF2 and H19 DMRs in the offspring by parental obesity. The graphs represent the mean estimated methylation values of 69 newborns at the IGF2 DMR, and 70 newborns at the H19 DMR. The IGF2 DMR results are based on 14 obese fathers and 25 obese mothers; the results at the H19 DMR are based on 15 obese fathers and 23 obese mothers. For each exposure the differences of the least square means of methylation percentages are shown at each CpG site (bold), as well as standard errors (SE) and _P_-values. Bars represent standard errors.
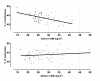
Figure 2
Offspring's mean methylation % at the IGF2 DMR by BMI of the parents. The predicted methylation means at IGF2 DMR are plotted by BMI of the father (upper graph), and BMI of the mother (lower graph); adjusted for maternal age, smoking status, BMI of the other parent, the newborn's birth weight and gender.
Comment in
- Fat dads must not be blamed for their children's health problems.
Moore GE, Stanier P. Moore GE, et al. BMC Med. 2013 Feb 6;11:30. doi: 10.1186/1741-7015-11-30. BMC Med. 2013. PMID: 23388448 Free PMC article.
Similar articles
- Newborns of obese parents have altered DNA methylation patterns at imprinted genes.
Soubry A, Murphy SK, Wang F, Huang Z, Vidal AC, Fuemmeler BF, Kurtzberg J, Murtha A, Jirtle RL, Schildkraut JM, Hoyo C. Soubry A, et al. Int J Obes (Lond). 2015 Apr;39(4):650-7. doi: 10.1038/ijo.2013.193. Epub 2013 Oct 25. Int J Obes (Lond). 2015. PMID: 24158121 Free PMC article. - Placenta-specific epimutation at H19-DMR among common pregnancy complications: its frequency and effect on the expression patterns of H19 and IGF2.
Yamaguchi Y, Tayama C, Tomikawa J, Akaishi R, Kamura H, Matsuoka K, Wake N, Minakami H, Kato K, Yamada T, Nakabayashi K, Hata K. Yamaguchi Y, et al. Clin Epigenetics. 2019 Aug 1;11(1):113. doi: 10.1186/s13148-019-0712-3. Clin Epigenetics. 2019. PMID: 31370882 Free PMC article. - Dietary fat intake during early pregnancy is associated with cord blood DNA methylation at IGF2 and H19 genes in newborns.
Chiu YH, Fadadu RP, Gaskins AJ, Rifas-Shiman SL, Laue HE, Moley KH, Hivert MF, Baccarelli A, Oken E, Chavarro JE, Cardenas A. Chiu YH, et al. Environ Mol Mutagen. 2021 Aug;62(7):388-398. doi: 10.1002/em.22452. Epub 2021 Jul 31. Environ Mol Mutagen. 2021. PMID: 34288135 Free PMC article. - Mechanisms of Igf2/H19 imprinting: DNA methylation, chromatin and long-distance gene regulation.
Sasaki H, Ishihara K, Kato R. Sasaki H, et al. J Biochem. 2000 May;127(5):711-5. doi: 10.1093/oxfordjournals.jbchem.a022661. J Biochem. 2000. PMID: 10788777 Review. - Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review.
Dunford AR, Sangster JM. Dunford AR, et al. Diabetes Metab Syndr. 2017 Dec;11 Suppl 2:S655-S662. doi: 10.1016/j.dsx.2017.04.021. Epub 2017 May 10. Diabetes Metab Syndr. 2017. PMID: 28533070 Review.
Cited by
- Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC).
Richmond RC, Simpkin AJ, Woodward G, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Smith AD, Timpson NJ, Tilling K, Davey Smith G, Relton CL. Richmond RC, et al. Hum Mol Genet. 2015 Apr 15;24(8):2201-17. doi: 10.1093/hmg/ddu739. Epub 2014 Dec 30. Hum Mol Genet. 2015. PMID: 25552657 Free PMC article. - Effects of endocrine disruptors on fetal testis development, male puberty, and transition age.
Cargnelutti F, Di Nisio A, Pallotti F, Sabovic I, Spaziani M, Tarsitano MG, Paoli D, Foresta C. Cargnelutti F, et al. Endocrine. 2021 May;72(2):358-374. doi: 10.1007/s12020-020-02436-9. Epub 2020 Aug 5. Endocrine. 2021. PMID: 32757113 Free PMC article. Review. - Precision medicine in adult and pediatric obesity: a clinical perspective.
Bomberg EM, Ryder JR, Brundage RC, Straka RJ, Fox CK, Gross AC, Oberle MM, Bramante CT, Sibley SD, Kelly AS. Bomberg EM, et al. Ther Adv Endocrinol Metab. 2019 Jul 27;10:2042018819863022. doi: 10.1177/2042018819863022. eCollection 2019. Ther Adv Endocrinol Metab. 2019. PMID: 31384417 Free PMC article. Review. - Association of blood leukocyte DNA methylation at LINE-1 and growth-related candidate genes with pubertal onset and progression.
Wu Y, Peterson KE, Sánchez BN, Dolinoy DC, Mercado-Garcia A, Téllez-Rojo MM, Goodrich JM. Wu Y, et al. Epigenetics. 2018;13(12):1222-1233. doi: 10.1080/15592294.2018.1556198. Epub 2018 Dec 22. Epigenetics. 2018. PMID: 30582410 Free PMC article. - Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds.
Pascoal GFL, Geraldi MV, Maróstica MR Jr, Ong TP. Pascoal GFL, et al. Nutrients. 2022 May 21;14(10):2150. doi: 10.3390/nu14102150. Nutrients. 2022. PMID: 35631291 Free PMC article. Review.
References
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21ES014947/ES/NIEHS NIH HHS/United States
- R25 CA126938/CA/NCI NIH HHS/United States
- R01DK085173/DK/NIDDK NIH HHS/United States
- R01 ES016772/ES/NIEHS NIH HHS/United States
- R01 DK085173/DK/NIDDK NIH HHS/United States
- R25CA126938-01A2/CA/NCI NIH HHS/United States
- R01ES016772/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous