Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice - PubMed (original) (raw)
Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice
Xian-Cang Ma et al. PLoS One. 2013.
Abstract
Background: Clinical studies demonstrate that the N-methyl-D-aspartate (NMDA) receptor antagonist, ketamine, induces rapid antidepressant effects in patients with refractive major depressive disorder and bipolar depression. This rapid onset of action makes ketamine a highly attractive drug for patients, particularly those who do not typically respond to therapy. A recent study suggested that glycogen synthase kinase (GSK)-3 may underlie the rapid antidepressant action of ketamine, although the precise mechanisms are unclear. In this study, we examined the effects of ketamine and GSK-3 inhibitor SB216763 in the unpredictable, chronic mild stress (CMS) mouse model of mice.
Methodology/principal findings: Adult C57/B6 male mice were divided into 2 groups, a non-stressed control group and the unpredictable CMS (35 days) group. Then, either vehicle, ketamine (10 mg/kg), or the established GSK-3 inhibitor, SB216763 (10 mg/kg), were administered into mice in the CMS group, while vehicle was administered to controls. In the open field test, there was no difference between the four groups (control+vehicle, CMS+vehicle, CMS+ketamine, CMS+SB216763). In the sucrose intake test, a 1% sucrose intake drop, seen in CMS mice, was significantly attenuated after a single dose of ketamine, but not SB216763. In the tail suspension test (TST) and forced swimming test (FST), the increased immobility time seen in CMS mice was significantly attenuated by a single dose of ketamine, but not SB216763. Interestingly, the ketamine-induced increase in the sucrose intake test persisted for 8 days after a single dose of ketamine. Furthermore, a single administration of ketamine, but not SB216763, significantly attenuated the immobility time of the TST and FST in the control (non-stressed) mice.
Conclusions/significance: These findings suggest that a single administration of ketamine, but not GSK-3 inhibitor SB216763, produces a long-lasting antidepressant action in CMS model mice.
Conflict of interest statement
Competing Interests: Kenji Hashimoto is a member of the Editorial Board of PLOS ONE. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
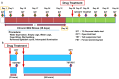
Figure 1. Experimental protocol.
(A): CMS paradigm. CMS procedures were performed for 5-weeks. Drug treatment was performed day 36. The 1% sucrose intake test (SIT) was performed at baseline, days 3, 10, 17, 24, 31, 37, 40, 42 and 44. Vehicle, ketamine (10 mg/kg), or SB216763 (10 mg/kg) were administered at day 36. The open field test (OFT) was performed at day 37. The tail suspension test (TST) and the forced swimming test (FST) was performed at day 38. (B): Acute effects of ketamine and SB216763 in control mice. Vehicle, ketamine (10 mg/kg, i.p.), or SB216763 (2.5, 5.0, or 10 mg/kg, i.p.) were administered into control mice. The behavioral tests, OFT, TST, and FST, were performed 3 and 24 hours after a single administration.
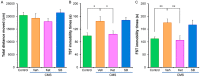
Figure 2. Effects of ketamine and the established GSK-3 inhibitor SB216763 in the CMS model.
(A) Locomotion: There were no differences between the four groups. Data show the mean±SEM (n = 8 or 9). (B) Tail-suspension test (TST): The increased immobility time of mice in the CMS groups, decreased significantly 48 hours (day 38) after a single dose of ketamine (10 mg/kg, i.p.), but not SB216763 (10 mg/kg, i.p.). Data show the mean±SEM (n = 5–8). (C) Forced swimming test (FST): The increased immobility time of mice in the CMS groups decreased significantly 48 hours (day 38) after a single dose of ketamine (10 mg/kg, i.p.), but not SB216763 (10 mg/kg, i.p.). Data show the mean±SEM (n = 8 or 9). *p<0.05, **p<0.01 as compared to CMS+Vehicle group.
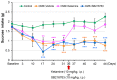
Figure 3. Effects of ketamine and the established GSK-3 inhibitor SB216763 in the anhedonia model.
The decreased intake of 1% sucrose in the CMS groups was significantly attenuated 24 hours, 4 days, 6 days and 8 days after a single dose of ketamine (10 mg/kg, i.p.), but not of SB216763 (10 mg/kg, i.p.). Data show the mean±SEM (n = 8 or 9). **p<0.01, ***p<0.001 as compared to Control group.
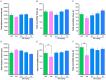
Figure 4. Effects of ketamine and SB216763 on control mice.
Behavioral tests in control mice were performed 3 hours and 24 hours after a single administration of vehicle, ketamine (10 mg/kg, i.p.) or SB216763 (2.5, 5.0, or 10 mg/kg, i.p.). (A): Locomotion: There were no differences between the five groups. Data show the mean±SEM (n = 14–16). (B) Tail-suspension test (TST): There were no differences between the five groups. Data show the mean±SEM (n = 13–16). (C) Forced swimming test (FST): There were no differences between the five groups. Data show the mean±SEM (n = 13–15). (D) Locomotion: There were no differences between the five groups. Data show the mean±SEM (n = 15 or 16). (E) Tail-suspension test (TST): Ketamine significantly (p = 0.001) decreased immobility time, 24 hours after administration. Data show the mean±SEM (n = 15 or 16). (C) Forced swimming test (FST): Ketamine significantly (p = 0.037) decreased immobility time, 24 hours after administration. Data show the mean±SEM (n = 15 or 16). *p<0.05, **p<0.01 as compared with the control group.
Similar articles
- The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress.
Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, Chuang DM. Chiu CT, et al. Int J Neuropsychopharmacol. 2014 Dec 28;18(6):pyu102. doi: 10.1093/ijnp/pyu102. Int J Neuropsychopharmacol. 2014. PMID: 25548109 Free PMC article. - Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and "depressed" mice exposed to chronic mild stress.
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Franceschelli A, et al. Neuroscience. 2015 Apr 2;290:49-60. doi: 10.1016/j.neuroscience.2015.01.008. Epub 2015 Jan 14. Neuroscience. 2015. PMID: 25595985 - GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine.
Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. Liu RJ, et al. Neuropsychopharmacology. 2013 Oct;38(11):2268-77. doi: 10.1038/npp.2013.128. Epub 2013 May 17. Neuropsychopharmacology. 2013. PMID: 23680942 Free PMC article. - A review of ketamine in affective disorders: current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action.
Naughton M, Clarke G, O'Leary OF, Cryan JF, Dinan TG. Naughton M, et al. J Affect Disord. 2014 Mar;156:24-35. doi: 10.1016/j.jad.2013.11.014. Epub 2013 Dec 10. J Affect Disord. 2014. PMID: 24388038 Review. - Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties.
Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Zunszain PA, et al. Mol Psychiatry. 2013 Dec;18(12):1236-41. doi: 10.1038/mp.2013.87. Epub 2013 Jul 23. Mol Psychiatry. 2013. PMID: 23877835 Free PMC article. Review.
Cited by
- Ketamine's rapid antidepressant effects are mediated by Ca2+-permeable AMPA receptors.
Zaytseva A, Bouckova E, Wiles MJ, Wustrau MH, Schmidt IG, Mendez-Vazquez H, Khatri L, Kim S. Zaytseva A, et al. Elife. 2023 Jun 26;12:e86022. doi: 10.7554/eLife.86022. Elife. 2023. PMID: 37358072 Free PMC article. - A single dose of ketamine enhances early life stress-induced aggression with no effect on fear memory, anxiety-like behavior, or depression-like behavior in mice.
Bartsch CJ, Aaflaq S, Jacobs JT, Smith M, Summa F, Skinner S, Qasem E, Thompson R, Li Z, Nordman JC. Bartsch CJ, et al. Behav Neurosci. 2023 Oct;137(5):281-288. doi: 10.1037/bne0000560. Epub 2023 Jun 15. Behav Neurosci. 2023. PMID: 37326523 Free PMC article. - Role of the mesolimbic dopamine pathway in the antidepressant effects of ketamine.
Cardona-Acosta AM, Bolaños-Guzmán CA. Cardona-Acosta AM, et al. Neuropharmacology. 2023 Mar 1;225:109374. doi: 10.1016/j.neuropharm.2022.109374. Epub 2022 Dec 11. Neuropharmacology. 2023. PMID: 36516891 Free PMC article. Review. - Participation of the Serotonergic System and Brain-Derived Neurotrophic Factor in the Antidepressant-like Effect of Flavonoids.
German-Ponciano LJ, Rosas-Sánchez GU, Cueto-Escobedo J, Fernández-Demeneghi R, Guillén-Ruiz G, Soria-Fregozo C, Herrera-Huerta EV, Rodríguez-Landa JF. German-Ponciano LJ, et al. Int J Mol Sci. 2022 Sep 17;23(18):10896. doi: 10.3390/ijms231810896. Int J Mol Sci. 2022. PMID: 36142808 Free PMC article. Review. - Encore: Behavioural animal models of stress, depression and mood disorders.
Petković A, Chaudhury D. Petković A, et al. Front Behav Neurosci. 2022 Aug 8;16:931964. doi: 10.3389/fnbeh.2022.931964. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36004305 Free PMC article. Review.
References
- Hashimoto K (2009) Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 61: 105–123. - PubMed
- Skolnick P, Popik P, Trullas R (2009) Glutamate-based antidepressants: 20 years on. Trends pharmacol Sci 30: 563–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was supported by a grant from the National Natural Science Foundation of China (#81171256 and #81171262) and a grant from the Minister of Education, Culture, Sports, Science, and Technology of Japan (to K.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous