Animals in a bacterial world, a new imperative for the life sciences - PubMed (original) (raw)
Review
. 2013 Feb 26;110(9):3229-36.
doi: 10.1073/pnas.1218525110. Epub 2013 Feb 7.
Michael G Hadfield, Thomas C G Bosch, Hannah V Carey, Tomislav Domazet-Lošo, Angela E Douglas, Nicole Dubilier, Gerard Eberl, Tadashi Fukami, Scott F Gilbert, Ute Hentschel, Nicole King, Staffan Kjelleberg, Andrew H Knoll, Natacha Kremer, Sarkis K Mazmanian, Jessica L Metcalf, Kenneth Nealson, Naomi E Pierce, John F Rawls, Ann Reid, Edward G Ruby, Mary Rumpho, Jon G Sanders, Diethard Tautz, Jennifer J Wernegreen
Affiliations
- PMID: 23391737
- PMCID: PMC3587249
- DOI: 10.1073/pnas.1218525110
Review
Animals in a bacterial world, a new imperative for the life sciences
Margaret McFall-Ngai et al. Proc Natl Acad Sci U S A. 2013.
Abstract
In the last two decades, the widespread application of genetic and genomic approaches has revealed a bacterial world astonishing in its ubiquity and diversity. This review examines how a growing knowledge of the vast range of animal-bacterial interactions, whether in shared ecosystems or intimate symbioses, is fundamentally altering our understanding of animal biology. Specifically, we highlight recent technological and intellectual advances that have changed our thinking about five questions: how have bacteria facilitated the origin and evolution of animals; how do animals and bacteria affect each other's genomes; how does normal animal development depend on bacterial partners; how is homeostasis maintained between animals and their symbionts; and how can ecological approaches deepen our understanding of the multiple levels of animal-bacterial interaction. As answers to these fundamental questions emerge, all biologists will be challenged to broaden their appreciation of these interactions and to include investigations of the relationships between and among bacteria and their animal partners as we seek a better understanding of the natural world.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
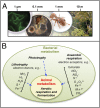
Animals through time. (A) Upper atmospheric oxygen concentration, as a percent of current levels, plotted against geological time. (B) Phylogenetic history of life on Earth, scaled to match the oxygen timeline. Note that the origin of the eukaryotes and the subsequent diversification of animals both correspond to periods of increasing atmospheric oxygen. (C) (Left) A phylogeny of choanoflagellates and selected animals, annotated to indicate the evolution of characters particularly relevant to interactions with bacteria. (Right) Interactions between bacteria and eukaryotes, corresponding to the phylogeny. Bacteria are prey, sources of metabolites, inducers of development in symbiosis (morphogenesis) and in larval settlement (environmental cues), and activators of immune systems.
Fig. 2.
The ancestry of humans reflected in the genomic signature. A phylogenetic analysis of the human genes reveals the relative percentage of the genome that arose at a series of stages in biological evolution (20).
Fig. 3.
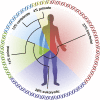
Signaling within and between the animal and its microbiota. Members of the microbiota, such as those in and on the gut, oral cavity, and skin, communicate among themselves and exchange signals with the animal’s organ systems, participating in the body’s homeostasis. Some of the signals promoting this balance are mentioned in the text (green), whereas other representatives are not (black;
Tables S1
and
S2
). The microbiota also influences animal behavior, creating a direct interface with other organisms. AMP, antimicrobial peptides; LPS, lipopolysaccharide; PGN, peptidoglycan; PSA, polysaccharide A; SCFA, short-chain fatty acids; TMA, trimethylamine oxide.
Fig. 4.
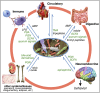
Nested ecological interactions of animals and bacteria and their underlying metabolic bases. (A) A forest canopy insect illustrates the cascading effects of animal-bacterial interactions across multiple spatial scales. Bacterial symbionts (Left), residing in the gut (Center Left), are essential to nutritional success of insect species (Center Right) in tropical forest canopies (Right), where they often make up a majority of animal biomass. (B) Diversity of energy metabolism in bacteria and animals. Animals can ferment and aerobically respire but are unable to perform the vast diversity of other, ecologically vital, energy-harvesting processes. Beyond phototrophy, which they share with plants, bacteria can also contribute to primary production by using inorganic energy sources (lithotrophy) to fix CO2. Animals are directly or indirectly dependent on bacteria for extracting energy and cycling biomolecules, whereas animals actively contribute to bacterial productivity through bioturbation, nutrient provisioning, and as habitats for colonization and shelter.
Similar articles
- Microbes in the coral holobiont: partners through evolution, development, and ecological interactions.
Thompson JR, Rivera HE, Closek CJ, Medina M. Thompson JR, et al. Front Cell Infect Microbiol. 2015 Jan 7;4:176. doi: 10.3389/fcimb.2014.00176. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25621279 Free PMC article. Review. - Strategies of genomic integration within insect-bacterial mutualisms.
Wernegreen JJ. Wernegreen JJ. Biol Bull. 2012 Aug;223(1):112-22. doi: 10.1086/BBLv223n1p112. Biol Bull. 2012. PMID: 22983037 Free PMC article. - Marine molecular biology: an emerging field of biological sciences.
Thakur NL, Jain R, Natalio F, Hamer B, Thakur AN, Müller WE. Thakur NL, et al. Biotechnol Adv. 2008 May-Jun;26(3):233-45. doi: 10.1016/j.biotechadv.2008.01.001. Epub 2008 Jan 26. Biotechnol Adv. 2008. PMID: 18299181 Review. - The Cost of Metabolic Interactions in Symbioses between Insects and Bacteria with Reduced Genomes.
Ankrah NYD, Chouaia B, Douglas AE. Ankrah NYD, et al. mBio. 2018 Sep 25;9(5):e01433-18. doi: 10.1128/mBio.01433-18. mBio. 2018. PMID: 30254121 Free PMC article.
Cited by
- Fecal Microbiota Transplantation Shows Marked Shifts in the Multi-Omic Profiles of Porcine Post-weaning Diarrhea.
Su Y, Li X, Li D, Sun J. Su Y, et al. Front Microbiol. 2021 Feb 23;12:619460. doi: 10.3389/fmicb.2021.619460. eCollection 2021. Front Microbiol. 2021. PMID: 33708182 Free PMC article. - Reflections on the Use of an Invertebrate Chordate Model System for Studies of Gut Microbial Immune Interactions.
Liberti A, Natarajan O, Atkinson CGF, Sordino P, Dishaw LJ. Liberti A, et al. Front Immunol. 2021 Feb 25;12:642687. doi: 10.3389/fimmu.2021.642687. eCollection 2021. Front Immunol. 2021. PMID: 33717199 Free PMC article. Review. - Locally adapted gut microbiomes mediate host stress tolerance.
Houwenhuyse S, Stoks R, Mukherjee S, Decaestecker E. Houwenhuyse S, et al. ISME J. 2021 Aug;15(8):2401-2414. doi: 10.1038/s41396-021-00940-y. Epub 2021 Mar 3. ISME J. 2021. PMID: 33658622 Free PMC article. - The Composition and Function of Pigeon Milk Microbiota Transmitted From Parent Pigeons to Squabs.
Ding J, Liao N, Zheng Y, Yang L, Zhou H, Xu K, Han C, Luo H, Qin C, Tang C, Wei L, Meng H. Ding J, et al. Front Microbiol. 2020 Aug 4;11:1789. doi: 10.3389/fmicb.2020.01789. eCollection 2020. Front Microbiol. 2020. PMID: 32849405 Free PMC article. - A targeted approach to enrich host-associated bacteria for metagenomic sequencing.
Dungan AM, Tandon K, Jameson V, Gotze CR, Blackall LL, van Oppen MJH. Dungan AM, et al. FEMS Microbes. 2023 Nov 28;5:xtad021. doi: 10.1093/femsmc/xtad021. eCollection 2024. FEMS Microbes. 2023. PMID: 38264162 Free PMC article.
References
- Gilbert SF, Sapp J, Tauber AI. A symbiotic view of life: We have never been individuals. Q Rev Biol. 2012;87(4):335–341. - PubMed
- Knoll AH. Life on a Young Planet. Princeton, NJ: Princeton Univ Press; 2003.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials