Nutrient sensing, metabolism, and cell growth control - PubMed (original) (raw)
Review
Nutrient sensing, metabolism, and cell growth control
Hai-Xin Yuan et al. Mol Cell. 2013.
Abstract
Cell growth is regulated by coordination of both extracellular nutrients and intracellular metabolite concentrations. AMP-activated kinase and mammalian target of rapamycin complex 1 serve as key molecules that sense cellular energy and nutrients levels, respectively. In addition, the members of the dioxygenase family, including prolylhydroxylase, lysine demethylase, and DNA demethylase, have emerged as possible sensors of intracellular metabolic status. The interplay among nutrients, metabolites, gene expression, and protein modification are involved in the coordination of cell growth with extracellular and intracellular conditions.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
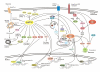
Figure 1
Nutrient sensing and metabolism pathways. AMP-activated kinase (AMPK) is activated by energy stress and promotes catabolic pathways to produce ATP while switching off ATP-consuming anabolic pathways. In contrast, mammalian target of rapamycin complex 1 (mTORC1) activation under nutrient-sufficiency leads to significant elevation of anabolic processes, such as protein and lipid synthesis. Abbreviations are: GLU4, glucose transporter type 4; CPT1, Carnitine Palmitoyltransferase-1; TAK1, TGFβ-activated kinase 1; LKB1, liver kinase B1 (a key AMPK activator tumor suppressor); CAMKKβ, Calmodulin-dependent protein kinase kinase β; ACC, acetyl CoA carboxylase; PFK2, Phosphofructokinase 2; GS, glycogen synthase; HDACs, histone deacetylases; CRTC2, CREB-regulated transcription co-activator 2; FOXO, forkhead box protein O; CREB, cAMP response element-binding protein; ULK, UNC-51-like kinase; FIP200, 200 kDa FAK family kinase-interacting protein; ATG, autophagy-related; PI3K, phosphoinositide 3-kinase; IRS1, insulin receptor substrate 1; PTEN, phosphatase and tensin homologue; PDK1, 3-phosphoinositide-dependent protein kinase 1; TSC1/2, tuberous sclerosis 1/2; Rheb, Ras homologue enriched in brain; v-ATPase, vacuolar H+-adenosine triphosphatase; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1; S6K, ribosomal S6 kinase; Ragulator, a protein complex responsible for lysosomal recruitment and activation of Rag GTPases; PGC1α, peroxisome proliferator-activated receptor-γ coactivator 1α; PPARγ, peroxisome proliferator-activated receptor-γ; SREBP, sterol regulatory element-binding protein; HIF, Hypoxia-inducible factors. Stimulatory interactions are indicated with ↓ and inhibitory interactions are indicated with ⊥.
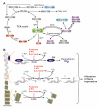
Figure 2
Dioxygenases in sensing metabolic intermediates and epigenetic regulation. (A) Schematics of α-KG metabolism and TCA cycle. The mutant isocitrate dehydrogenase (IDH) (indicated with a star) may decrease α-KG and generate a new oncometabolite 2-hydroxylglutarate (2-HG). (B) A proposed role of dioxygenases in metabolite sensing and epigenetic modifications. Using α-KG as a key substrate, dioxygenases is involved in HIFα hydroxylation, DNA demethylation, and histone demethylation. All processes are inhibited by normal metabolites, such as succinate and fumarate, as well as oncometabolite 2-HG. α-KG, α-ketoglutarate; GDH, glutamate dehydrogenase; SDH, succinate dehydrogenase; FH, fumarase; TET, ten-eleven translocation; KDM, lysine demethylase; PHD, prolyl hydroxylase domain protein.
Similar articles
- AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control.
González A, Hall MN, Lin SC, Hardie DG. González A, et al. Cell Metab. 2020 Mar 3;31(3):472-492. doi: 10.1016/j.cmet.2020.01.015. Cell Metab. 2020. PMID: 32130880 Review. - LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.
Shaw RJ. Shaw RJ. Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review. - Cross-talk between AMPK and mTOR in regulating energy balance.
Xu J, Ji J, Yan XH. Xu J, et al. Crit Rev Food Sci Nutr. 2012;52(5):373-81. doi: 10.1080/10408398.2010.500245. Crit Rev Food Sci Nutr. 2012. PMID: 22369257 Review. - Metabolic sensors and their interplay with cell signalling and transcription.
Krejčí A. Krejčí A. Biochem Soc Trans. 2012 Apr;40(2):311-23. doi: 10.1042/BST20110767. Biochem Soc Trans. 2012. PMID: 22435805 Review. - The role of LKB1 and AMPK in cellular responses to stress and damage.
Alexander A, Walker CL. Alexander A, et al. FEBS Lett. 2011 Apr 6;585(7):952-7. doi: 10.1016/j.febslet.2011.03.010. Epub 2011 Mar 12. FEBS Lett. 2011. PMID: 21396365 Review.
Cited by
- Bacillus anthracis Prolyl 4-Hydroxylase Modifies Collagen-like Substrates in Asymmetric Patterns.
Schnicker NJ, Dey M. Schnicker NJ, et al. J Biol Chem. 2016 Jun 17;291(25):13360-74. doi: 10.1074/jbc.M116.725432. Epub 2016 Apr 21. J Biol Chem. 2016. PMID: 27129244 Free PMC article. - Tumor Dormancy and Reactivation: The Role of Heat Shock Proteins.
Amissah HA, Combs SE, Shevtsov M. Amissah HA, et al. Cells. 2024 Jun 23;13(13):1087. doi: 10.3390/cells13131087. Cells. 2024. PMID: 38994941 Free PMC article. Review. - Duck gut metagenome reveals the microbiome signatures linked to intestinal regional, temporal development, and rearing condition.
Ma L, Lyu W, Zeng T, Wang W, Chen Q, Zhao J, Zhang G, Lu L, Yang H, Xiao Y. Ma L, et al. Imeta. 2024 May 14;3(4):e198. doi: 10.1002/imt2.198. eCollection 2024 Aug. Imeta. 2024. PMID: 39135685 Free PMC article. - K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells.
Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. Linares JF, et al. Mol Cell. 2013 Aug 8;51(3):283-96. doi: 10.1016/j.molcel.2013.06.020. Epub 2013 Aug 1. Mol Cell. 2013. PMID: 23911927 Free PMC article. - The Cutting Edge: The Role of mTOR Signaling in Laminopathies.
Chiarini F, Evangelisti C, Cenni V, Fazio A, Paganelli F, Martelli AM, Lattanzi G. Chiarini F, et al. Int J Mol Sci. 2019 Feb 15;20(4):847. doi: 10.3390/ijms20040847. Int J Mol Sci. 2019. PMID: 30781376 Free PMC article. Review.
References
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. - PubMed
- Binda M, Peli-Gulli MP, Bonfils G, Panchaud N, Urban J, Sturgill TW, Loewith R, De Virgilio C. The Vam6 GEF controls TORC1 by activating the EGO complex. Mol Cell. 2009;35:563–573. - PubMed
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. - PubMed
- Browne GJ, Finn SG, Proud CG. Stimulation of the AMP-activated protein kinase leads to activation of eukaryotic elongation factor 2 kinase and to its phosphorylation at a novel site, serine 398. J Biol Chem. 2004;279:12220–12231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources