Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin - PubMed (original) (raw)
Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin
Wenling Li et al. Dev Cell. 2013.
Abstract
In developing limb skin, peripheral nerves provide a spatial template that controls the branching pattern and differentiation of arteries. Our previous studies indicate that nerve-derived VEGF-A is required for arterial differentiation but not for nerve-vessel alignment. In this study, we demonstrate that nerve-vessel alignment depends on the activity of Cxcl12-Cxcr4 chemokine signaling. Genetic inactivation of Cxcl12-Cxcr4 signaling perturbs nerve-vessel alignment and abolishes arteriogenesis. Further in vitro assays allow us to uncouple nerve-vessel alignment and arteriogenesis, revealing that nerve-derived Cxcl12 stimulates endothelial cell migration, whereas nerve-derived VEGF-A is responsible for arterial differentiation. These findings suggest a coordinated sequential action in which nerve Cxcl12 functions over a distance to recruit vessels to align with nerves, and subsequent arterial differentiation presumably requires a local action of nerve VEGF-A in the nerve-associated vessels.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
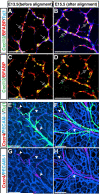
Figure 1. Complementary expression of Cxcl12 and its receptor Cxcr4 in nerves and vessels in the developing limb skin
Whole-mount immunohistochemical analysis of limb skin from E13.5 and E15.5 Cxcl12GFP embryos with antibodies to the neuron specific marker βIII tubulin (Tuj1, A and B, blue, and E and F, green), the Schwann cell marker BFABP (A-D, red), the pan-endothelial cell marker PECAM-1 (E-H, blue), Cxcl12 (Cxcl12GFP, A-D, green) and Cxcr4 (anti-Cxcr4 antibody from Biotrend, E-H, red) is shown. At E13.5, no association between nerves and capillary vessels was yet evident (E). BFABP+ migrating Schwann cells in nerves express Cxcl12 (A and C, open arrows). Cxcr4 expression was detected in a subset of capillary vessels near nerves (E and G, red, arrowheads). By E15.5, vascular remodeling had occurred, and nerves were associated with arteries (F). Schwann cells continued to express Cxcl12 (B and D, open arrows), whereas Cxcr4 expression was restricted to nerve-associated arteries (F and H, arrowheads). Scale bars are 100 μm.
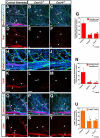
Figure 2. Disruption of nerve-vessel alignment in Cxcl12 and Cxcr4 homozygous mutants
Whole-mount double immunofluorescence labeling of limb skin with antibodies to PECAM-1 (A-C and G-I, blue; D-F and J-L, white) and βIII tubulin (Tuj1, A-C and G-I, green) in Cxcl12-/- mutants (B, E, H, K), Cxcr4-/- mutants (C, F, I, L), or control littermates (A, D, G, J) at E15.5 is shown. Close-up images (G-L) show the boxed regions in A, B, C. Both Cxcl12-/- and Cxcr4-/- mutants exhibited aberrant patterns of remodeled vessels (B, E, H, K for Cxcl12-/- mutants; C, F, I, L for Cxcr4-/- mutants, arrowheads), resulting in disruption of nerve-vessel alignment (B, C, H, I, open arrows and arrowheads). Note that branching patterns of Tuj1+ nerves are not affected in either homozygous mutant (A-C and G-I, green). (M) Quantification of the nerve-vessel alignment as the percentage of nerve-length aligned with vessels was performed. Cxcl12-/- and Cxcr4-/- mutants exhibit defective nerve-vessel alignment. Asterisk indicates statistically significant difference (P<0.05) in both mutants compared with control littermates according to Student's _t_-test (n=5 per genotype; bars represent mean ± SEM). (N) Quantification of vascular density in Cxcl12-/- and Cxcr4-/- mutants compared with control littermates was shown (n=4 per genotype; bars represent mean ± SEM). There was no significant difference in vascular density between Cxcl12-/- mutants, Cxcr4-/- mutants, and control littermates. Scale bars are 100 μm.
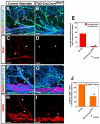
Figure 3. Defective arterial differentiation and smooth muscle coverage in Cxcl12 and Cxcr4 homozygous mutants
Whole-mount triple immunofluorescence labeling of limb skin with antibodies to arterial markers either Nrp1 (A-F, red, arrowheads) or Cx40 (H-M, red, arrowheads) and the smooth muscle cell marker αSMA (O-T, red, arrowheads), in addition to PECAM-1 (A-C, H-J and O-Q, blue) and neurofilament (2H3, A-C, H-J and O-Q, green, open arrows) in Cxcl12-/- (B, E, I, L, P, S), Cxcr4-/- (C, F, J, M, Q, T) or control littermates (A, D, H, K, O, R) at E15.5 is shown. The expression of arterial markers such as Nrp1 and Cx40 was conspicuously reduced in remodeled vessels of both Cxcl12-/- and Cxcr4-/- mutants (B, C, E, F for Nrp1 expression; I, J, L, M for Cx40 expression, arrowheads). Note that weak Nrp1 expression can be detected in some remodeled vessels in relatively close proximity to nerves in Cxcl12-/- (B, E, open arrowheads) and Cxcr4-/- mutants (C, F, open arrowheads). Fewer αSMA+ smooth muscle cells associated with small-diameter branched vessels in Cxcl12-/- and Cxcr4-/- mutants (O versus P and Q, R versus S and T, arrowheads), although smooth muscle coverage in large-diameter veins appeared unaffected (S and T, open arrowheads). Quantification of arterial marker expression (G and N) or αSMA+ smooth muscle cell coverage (U) in small-diameter branched vessels was performed (n=4 per genotype; bars represent mean ± SEM). Asterisk indicates statistically significant difference (P<0.05) in both mutants compared with control littermates according to Student's _t_-test. Scale bars are 100 μm.
Figure 4. Requirement for endothelial Cxcr4 for patterns of vascular branching and arteriogenesis
Whole-mount triple immunofluorescence labeling of limb skin with antibodies to the arterial marker Nrp1 (A-D, red, arrowheads) and the smooth muscle cell marker αSMA (F-I, red, arrowheads), in addition to PECAM-1 (A, B, F, G, blue) and neurofilament (2H3, A, B, F, G, green, open arrows) in Tie2-Cre; Cxcr4flox/- and control littermates is shown. Compared to control littermates, disrupted nerve-vessel alignment was observed in Tie2-Cre; Cxcr4flox/- mutants (A versus B, arrowheads and open arrows). The expression of Nrp1 was nearly abolished in Tie2-Cre; Cxcr4flox/- mutants (D, arrowheads). αSMA+ smooth muscle cell coverage was strongly reduced in small-diameter branched vessels in Tie2-Cre; Cxcr4flox/- mutants (F versus G, H versus I, arrowheads), although smooth muscle coverage in large-diameter veins appeared unaffected (F versus G, H versus I). Overall, Tie2-Cre; Cxcr4flox/- mutants exhibit an almost identical phenotype to Cxcl12-/- or Cxcr4-/- mutants. Quantification of Nrp1 expression (E) or αSMA+ smooth muscle cell coverage (J) in small-diameter branched vessels was performed (n=3 per genotype; bars represent mean ± SEM). Asterisk indicates statistically significant difference (P<0.05) in mutants compared with control littermates according to Student's _t_-test. Scale bars are 100 μm.
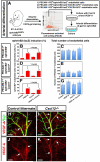
Figure 5. Cxcl12 cannot induce arterial differentiation in vitro
(A) Schematic illustrating the experimental procedure for endothelial cell isolation from E11.5 ephrinB2taulacZ/+ heterozygous embryos and the arterial differentiation assay in culture. (B-G) PECAM-1+/ephrinB2- (B and C), PECAM-1+/ephrinB2-/CXCR4+ (D and E) or PECAM-1+/ephrinB2-/CXCR4- (F and G) endothelial cells were cultured in 10 ng/ml VEGF-A, 500 ng/ml Cxcl12 or both proteins plus 10 ng/ml bFGF for 2 days, followed by double-staining with X-gal and anti-PECAM-1 antibody. Note that VEGF-A induced ephrinB2 expression in ~45% of FACS-purified endothelial cells without change in the total number of endothelial cells. By contrast, Cxcl12 by itself did not up-regulate ephrinB2 expression, and showed no synergistic effect with VEGF-A. Furthermore, no increase of ephrinB2 expression was observed under these conditions in the Cxcr4+ subpopulation of endothelial cells. Statistical analysis is from 3 independent experiments (bars represent mean ± SEM). Asterisk indicates statistically significant difference (P<0.05) according to Student's _t_-test. (H-K) Whole-mount immunohistochemical analysis of limb skin with antibodies to VEGF (red, arrows) and βIII tubulin (Tuj1, green) is shown. There was no significant difference in VEGF-A expression between Cxcl12-/- mutants (I and K) and control littermates (H and J). Scale bars are 100 μm.
Figure 6. Purified DRG sensory neurons and Schwann cells promote endothelial cell migration in vitro via Cxcl12-Cxcr4 signaling
(A) Schematic illustrating the experimental procedure for DRG dissection from E13.5 embryos and the chemotaxis assay. (B) DRG culture supernatant stimulated migration of Cxcr4+ MSS31 endothelial cells and this effect was blocked by treatment with either anti-Cxcr4 blocking antibody (R&D) or AMD3100, a selective antagonist for Cxcr4. 300 ng/ml Cxcl12 stimulated migration of MSS31 endothelial cells at a level similar to the DRG culture supernatant. Statistical analysis is from 3 independent experiments (bars represent mean ± SEM). Asterisks indicate statistically significant induction of migration of MSS31 endothelial cells (p<0.01) after treatment with Cxcl12 or the DRG culture supernatant, and statistically significant inhibition of DRG-supernatant stimulated migration of MSS31 endothelial cells (p<0.01) after treatment with anti-Cxcr4 blocking antibody or AMD3100, according to a Student's _t_-test. (C and D) The supernatant from Cxcl12 knock-down DRG culture failed to induce migration of MSS31 endothelial cells. DRGs were infected with a control or Cxcl12 shRNA lentivirus. The expression levels of Cxcl12 were assessed by RT-PCR analysis (C). Compared to the supernatant from control shRNA-treated DRG culture, the supernatant from Cxcl12 knock-down DRG culture failed to induce migration of MSS31 endothelial cells. Statistical analysis is from 3 independent experiments (bars represent mean ± SEM). Asterisks indicate statistically significant induction of migration of MSS31 endothelial cells (P<0.01) after treatment with the supernatant from control shRNA-treated DRG culture, and statistically significant reduction of migration of MSS31 endothelial cells (P<0.01) after treatment with the supernatant from Cxcl12 knock-down DRG culture, according to a Student's _t_-test. (E) The effect on endothelial cell migration induced by the DRG culture supernatant or 300ng/ml Cxcl12 was blocked by treatment with 2ng/ml Pertussis toxin, a G-protein inhibitor. Statistical analysis is from 3 independent experiments (bars represent mean ± SEM). Asterisk indicates statistically significant difference (P<0.01) according to Student's t-test. (F) VEGF-A is not responsible for the effect on endothelial cell migration induced by the DRG culture supernatant. 10ng/ml VEGF-A stimulated migration of MSS31 endothelial cells and this effect was blocked by treatment with 100 ng/ml soluble Flt1-Fc (VEGFR1-Fc) protein. 100ng/ml soluble Flt1-Fc protein failed to block DRG-supernatant stimulated migration of MSS31 endothelial cells. Statistical analysis is from 3 independent experiments (bars represent mean ± SEM). On the left panel, asterisks indicate statistically significant induction of migration of MSS31 endothelial cells (p<0.05) induced by treatment with VEGF-A, and statistically significant inhibition of VEGF-A stimulated migration of MSS31 endothelial cells (p<0.01) after treatment with soluble Flt1-Fc protein compared to control Fc protein, according to a Student's _t_-test. On the right panel, asterisks indicate statistically significant induction of migration of MSS31 endothelial cells (p<0.05) induced by treatment with the DRG culture supernatant with or without control Fc protein or soluble Flt1-Fc protein compared to basic medium, according to a Student's _t_-test.
Figure 7. Coordinate action of nerve-derived Cxcl12 and VEGF-A results in nerve-artery alignment
(A) Schematic model for nerve-mediated vascular branching and arterial differentiation in developing limb skin. Sensory nerves invade at approximately E11.5~13.5, after a primary capillary plexus is established. Subsequently, the pattern of sensory nerves provides signals (Cxcl12 and VEGF-A) that govern patterns of vascular branching and arterial differentiation during vascular remodeling. As a result, the congruence of blood vessel and nerve patterns is established in the skin. (B) This scheme shows how nerve-derived Cxcl12 and VEGF-A control patterns of vascular branching and arterial differentiation. In this view, oxygen-starved nerves may induce Cxcl12 and VEGF-A expression through activation of HIF-1 prior to vascular remodeling. Cxcl12-Cxcr4 signaling functions as a long-range chemotactic guidance cue to recruit vessels to align with nerves. Nerve-derived VEGF-A instructs arterial differentiation in the nerve-associated vessels. Arterial differentiation presumably requires a local-action of VEGF-A to induce arterial marker expression.
Comment in
- Nerve control of blood vessel patterning.
Makita T. Makita T. Dev Cell. 2013 Feb 25;24(4):340-1. doi: 10.1016/j.devcel.2013.02.003. Dev Cell. 2013. PMID: 23449469 Free PMC article.
Similar articles
- Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin.
Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Mukouyama YS, et al. Cell. 2002 Jun 14;109(6):693-705. doi: 10.1016/s0092-8674(02)00757-2. Cell. 2002. PMID: 12086669 - Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback.
Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Mukouyama YS, et al. Development. 2005 Mar;132(5):941-52. doi: 10.1242/dev.01675. Epub 2005 Jan 26. Development. 2005. PMID: 15673567 - Stromal-derived factor-1α/CXCL12-CXCR4 chemotactic pathway promotes perineural invasion in pancreatic cancer.
Xu Q, Wang Z, Chen X, Duan W, Lei J, Zong L, Li X, Sheng L, Ma J, Han L, Li W, Zhang L, Guo K, Ma Z, Wu Z, Wu E, Ma Q. Xu Q, et al. Oncotarget. 2015 Mar 10;6(7):4717-32. doi: 10.18632/oncotarget.3069. Oncotarget. 2015. PMID: 25605248 Free PMC article. - Regulation of endothelial cell differentiation and arterial specification by VEGF and Notch signaling.
Hirashima M. Hirashima M. Anat Sci Int. 2009 Sep;84(3):95-101. doi: 10.1007/s12565-009-0026-1. Epub 2009 Mar 4. Anat Sci Int. 2009. PMID: 19259767 Review. - CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization.
Liekens S, Schols D, Hatse S. Liekens S, et al. Curr Pharm Des. 2010;16(35):3903-20. doi: 10.2174/138161210794455003. Curr Pharm Des. 2010. PMID: 21158728 Review.
Cited by
- Whole-Mount Adult Ear Skin Imaging Reveals Defective Neuro-Vascular Branching Morphogenesis in Obese and Type 2 Diabetic Mouse Models.
Yamazaki T, Li W, Yang L, Li P, Cao H, Motegi SI, Udey MC, Bernhard E, Nakamura T, Mukouyama YS. Yamazaki T, et al. Sci Rep. 2018 Jan 11;8(1):430. doi: 10.1038/s41598-017-18581-7. Sci Rep. 2018. PMID: 29323138 Free PMC article. - Ablation of sensory nerves favours melanoma progression.
Prazeres PHDM, Leonel C, Silva WN, Rocha BGS, Santos GSP, Costa AC, Picoli CC, Sena IFG, Gonçalves WA, Vieira MS, Costa PAC, Campos LMCC, Lopes MTP, Costa MR, Resende RR, Cunha TM, Mintz A, Birbrair A. Prazeres PHDM, et al. J Cell Mol Med. 2020 Sep;24(17):9574-9589. doi: 10.1111/jcmm.15381. Epub 2020 Jul 20. J Cell Mol Med. 2020. PMID: 32691511 Free PMC article. - CXCL12 Signaling Is Essential for Maturation of the Ventricular Coronary Endothelial Plexus and Establishment of Functional Coronary Circulation.
Cavallero S, Shen H, Yi C, Lien CL, Kumar SR, Sucov HM. Cavallero S, et al. Dev Cell. 2015 May 26;33(4):469-77. doi: 10.1016/j.devcel.2015.03.018. Dev Cell. 2015. PMID: 26017771 Free PMC article. - Abnormal Crosstalk between Endothelial Cells and Podocytes Mediates Tyrosine Kinase Inhibitor (TKI)-Induced Nephrotoxicity.
Gu X, Zhang S, Zhang T. Gu X, et al. Cells. 2021 Apr 12;10(4):869. doi: 10.3390/cells10040869. Cells. 2021. PMID: 33921219 Free PMC article. Review. - Osteoblasts pattern endothelium and somatosensory axons during zebrafish caudal fin organogenesis.
Bump RG, Goo CEA, Horton EC, Rasmussen JP. Bump RG, et al. Development. 2022 Feb 1;149(3):dev200172. doi: 10.1242/dev.200172. Epub 2022 Feb 7. Development. 2022. PMID: 35129199 Free PMC article.
References
- Ara T, Tokoyoda K, Okamoto R, Koni PA, Nagasawa T. The role of CXCL12 in the organ specific process of artery formation. Blood. 2005;105:3155–3161. - PubMed
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. - PubMed
- Argraves WS, Drake CJ. Genes critical to vasculogenesis as defined by systematic analysis of vascular defects in knockout mice. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2005;286:875–884. - PubMed
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases