Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis - PubMed (original) (raw)
Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis
Wan-fu Wu et al. Proc Natl Acad Sci U S A. 2013.
Abstract
A therapeutic goal in the treatment of certain CNS diseases, including multiple sclerosis, amyotrophic lateral sclerosis, and Parkinson disease, is to down-regulate inflammatory pathways. Inflammatory molecules produced by microglia are responsible for removal of damaged neurons, but can cause collateral damage to normal neurons located close to defective neurons. Although estrogen can inactivate microglia and inhibit the recruitment of T cells and macrophages into the CNS, there is controversy regarding which of the two estrogen receptors (ERs), ERα or ERβ, mediates the beneficial effects in microglia. In this study, we found that ERβ, but not ERα, is expressed in microglia. Using the experimental autoimmune encephalomyelitis (EAE) model in SJL/J mice, we evaluated the benefit of an ERβ agonist as a modulator of neuroinflammation. Treatment of EAE mice with LY3201, a selective ERβ agonist provided by Eli Lilly, resulted in marked reduction of activated microglia in the spinal cord. LY3201 down-regulated the nuclear transcription factor NF-κB, as well as the NF-κB-induced gene inducible nitric oxide synthase in microglia and CD3(+) T cells. In addition, LY3201 inhibited T-cell reactivity through regulation of indoleamine-2,3-dioxygenase. In the EAE model, treatment with LY3201 decreased mortality in the first 2 wk after disease onset, and also reduced the severity of symptoms in mice surviving for 4 wk. Our data show that ERβ-selective agonists, by modulating the immune system in both microglia and T cells, offer promise as a useful class of drugs for treating degenerative diseases of the CNS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
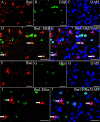
Expression of ERα and ERβ in microglia. (A–E) Iba1 and ERβ double-fluorescence staining. (D) Colocalization of ERβ (green) and Iba1 (red) (arrows). (E) Colocalization of ERβ (green), Iba1 (red), and DAPI (blue) (arrows). (F–J) ERα and Iba1 double-fluorescence staining. (I and J) Lack of colocalization between ERα (green) and Iba1 (red). The arrows show ERα-negative microglia. Nuclei were counterstained with DAPI (blue). (Scale bars: 20 μm.)
Fig. 2.
Microglial cells were inactivated in the spinal cord of EAE mice treated with LY3201. (A and B) In noninduced mice treated with LY3201, there were very few Iba1-positive resting microglial cells (arrow) in the spinal cord. (C, D, and G) EAE mice treated with vehicle had more Iba1-positive cells (**P < 0.01) than noninduced mice treated with LY3201 both in white matter and in gray matter of the spinal cord. These activated cells had larger cell bodies and ramified morphology (arrowheads). (E, F, and G) EAE mice treated with LY3201 had fewer Iba1-positive cells in the spinal cord (**P < 0.010) compared with vehicle-treated EAE mice, and these Iba1-positive cells had small cell bodies and long, thin processes. (H) Hematoxylin staining shows the white matter and the gray matter of the spinal cord. (Scale bars: 50 μm.)
Fig. 3.
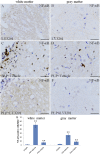
Down-regulation of activated NF-κB in the spinal cord of EAE mice treated with LY3201. (A and B) There were few NF-κB–positive cells in the spinal cord of noninduced mice treated with LY3201. (C, D, and G) There were more NF-κB–positive cells in vehicle-treated EAE mice (**P < 0.01) than in noninduced mice treated with LY3201. (E, F, and G) There were fewer NF-κB–positive cells in the spinal cords of EAE mice treated with LY3201 (**P < 0.01) than in vehicle-treated EAE mice. (Scale bars: 50 μm.)
Fig. 4.
Expression of NF-κB in microglia and T cells. NF-κB/Iba1 (A–E) and NF-κB/CD3 (F–J) double-fluorescence staining in gray matter of spinal cord of vehicle-treated EAE mice. (D) Colocalization of NF-κB (green) and Iba1 (red) (arrows). (E) Colocalization of NF-κB (green), Iba1 (red), and DAPI (blue) (arrows). (I) Colocalization of NF-κB (green) and CD3 (red) (arrows). (J) Colocalization of NF-κB (green), CD3 (red), and DAPI (blue) (arrows). (Scale bars: 20 μm.)
Fig. 5.
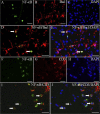
Reduced expression of iNOS in the spinal cord of LY3201-treated EAE mice. (A) There were very few iNOS-positive cells in the spinal cord of noninduced mice treated with LY3201. (B) In vehicle-treated EAE mice there was a markedly increased iNOS expression, especially in white matter. (C) The expression of iNOS was lower in LY3201-treated EAE mice than in vehicle-treated EAE mice. (D_–_H) iNOS and Iba1 double-fluorescence staining in white matter of the spinal cord of vehicle-treated EAE mice. (G) Colocalization of iNOS (green) and Iba1 (red) (arrows). (H) Colocalization of iNOS (green), Iba1 (red), and DAPI (blue) (arrows). (I_–_M) iNOS and CD3 double-fluorescence staining in white matter of spinal cord of vehicle-treated EAE mice. (L) Colocalization of iNOS (green) and CD3 (red) (arrows). (M) Colocalization of iNOS (green), CD3 (red), and DAPI (blue) (arrows). (Scale bars: 50 μm in A–C; 20 μm in D–M.)
Fig. 6.
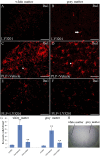
Fewer IDO-positive cells in the spinal cord of EAE mice treated with LY3201. (A) There were no IDO-positive cells in the spinal cord of noninduced mice treated with LY3201. (B) IDO-positive cells (red arrow) were markedly increased in vehicle-treated EAE mice. (C) There were fewer IDO-positive cells in the spinal cord of EAE mice treated with LY3201. (D) The number of IDO-positive cells differed significantly in LY3201-treated and vehicle-treated EAE mice. **P < 0.01. (Scale bars: 50 μm.)
Fig. 7.
LY3201 promoted recovery and decreased mortality in EAE mice. EAE score was determined daily for 30 d after PLP immunization. (A) Compared with vehicle-treated mice, LY3201-treated mice had less severe symptoms at 4 wk after PLP induction (*P < 0.05). (B) Treatment with LY3201 reduced the mortality of EAE mice in the first 2 wk. *P < 0.05.
Similar articles
- Sodium phenylacetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps.
Dasgupta S, Zhou Y, Jana M, Banik NL, Pahan K. Dasgupta S, et al. J Immunol. 2003 Apr 1;170(7):3874-82. doi: 10.4049/jimmunol.170.7.3874. J Immunol. 2003. PMID: 12646656 - Activation of Glucagon-Like Peptide-1 Receptor Promotes Neuroprotection in Experimental Autoimmune Encephalomyelitis by Reducing Neuroinflammatory Responses.
Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW. Lee CH, et al. Mol Neurobiol. 2018 Apr;55(4):3007-3020. doi: 10.1007/s12035-017-0550-2. Epub 2017 Apr 29. Mol Neurobiol. 2018. PMID: 28456941 - The benefits and detriments of macrophages/microglia in models of multiple sclerosis.
Rawji KS, Yong VW. Rawji KS, et al. Clin Dev Immunol. 2013;2013:948976. doi: 10.1155/2013/948976. Epub 2013 Jun 12. Clin Dev Immunol. 2013. PMID: 23840244 Free PMC article. Review. - Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration.
Spence RD, Voskuhl RR. Spence RD, et al. Front Neuroendocrinol. 2012 Jan;33(1):105-15. doi: 10.1016/j.yfrne.2011.12.001. Epub 2011 Dec 24. Front Neuroendocrinol. 2012. PMID: 22209870 Free PMC article. Review.
Cited by
- Dynamic Responses of Microglia in Animal Models of Multiple Sclerosis.
Plastini MJ, Desu HL, Brambilla R. Plastini MJ, et al. Front Cell Neurosci. 2020 Aug 20;14:269. doi: 10.3389/fncel.2020.00269. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32973458 Free PMC article. - ERβ mediates sex-specific protection in the App-NL-G-F mouse model of Alzheimer's disease.
Demetriou A, Lindqvist B, Ali HG, Shamekh MM, Maioli S, Inzunza J, Varshney M, Nilsson P, Nalvarte I. Demetriou A, et al. bioRxiv [Preprint]. 2024 Jul 23:2024.07.22.604543. doi: 10.1101/2024.07.22.604543. bioRxiv. 2024. PMID: 39091856 Free PMC article. Updated. Preprint. - ERβ-selective agonist alleviates inflammation in a multiple sclerosis model via regulation of MHC II in microglia.
Liu X, Deng J, Li R, Tan C, Li H, Yang Z, Chen L, Chen Y, Tan X. Liu X, et al. Am J Transl Res. 2019 Jul 15;11(7):4411-4424. eCollection 2019. Am J Transl Res. 2019. PMID: 31396345 Free PMC article. - Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair.
Larson TA. Larson TA. Front Endocrinol (Lausanne). 2018 Apr 30;9:205. doi: 10.3389/fendo.2018.00205. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29760681 Free PMC article. Review. - Estrogen Receptor β as a Candidate Regulator of Sex Differences in the Maternal Immune Activation Model of ASD.
Arnold ML, Saijo K. Arnold ML, et al. Front Mol Neurosci. 2021 Aug 31;14:717411. doi: 10.3389/fnmol.2021.717411. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34531723 Free PMC article. Review.
References
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. - PubMed
- Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3(2):104–110. - PubMed
- Vukusic S, Confavreux C. Primary and secondary progressive multiple sclerosis. J Neurol Sci. 2003;206(2):153–155. - PubMed
- Wingerchuk DM. Current evidence and therapeutic strategies for multiple sclerosis. Semin Neurol. 2008;28(1):56–68. - PubMed
- Wolinsky JS, et al. PROMiSe Trial Study Group Glatiramer acetate in primary progressive multiple sclerosis: Results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61(1):14–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases