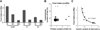High carbohydrate-low protein consumption maximizes Drosophila lifespan - PubMed (original) (raw)
High carbohydrate-low protein consumption maximizes Drosophila lifespan
Kimberley D Bruce et al. Exp Gerontol. 2013 Oct.
Abstract
Dietary restriction extends lifespan in a variety of organisms, but the key nutritional components driving this process and how they interact remain uncertain. In Drosophila, while a substantial body of research suggests that protein is the major dietary component affecting longevity, recent studies claim that carbohydrates also play a central role. To clarify how nutritional factors influence longevity, nutrient consumption and lifespan were measured on a series of diets with varying yeast and sugar content. We show that optimal lifespan requires both high carbohydrate and low protein consumption, but neither nutrient by itself entirely predicts lifespan. Increased dietary carbohydrate or protein concentration does not always result in reduced feeding-the regulation of food consumption is best described by a constant daily caloric intake target. Moreover, due to differences in food intake, increased concentration of a nutrient within the diet does not necessarily result in increased consumption of that particular nutrient. Our results shed light on the issue of dietary effects on lifespan and highlight the need for accurate measures of nutrient intake in dietary manipulation studies.
Keywords: Aging; C:P; Dietary restriction; Drosophila; Feeding; Longevity; Nutrition; carbohydrate protein ratio.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1
Relationship between lifespan and macronutrient consumption. (A) Effects of protein and carbohydrate intake on median lifespan of Canton-S (left) and Dahomey (right) male flies. Protein and carbohydrate consumption are determined from accumulation of radiolabeled food over 24 h. Circle size scales with increasing longevity and the median lifespan at each point of protein and carbohydrate ingestion is shown. Longevity is maximized at an optimal C:P between 10:1 and 20:1, in agreement with previous studies on female Canton-S flies (Lee et al. 2008) that identified the optimum at 16:1 (solid line). Other carbohydrate:protein rails (1:1, 2:1, 4:1, 8:1, and 32:1) are shown as dashed lines. (B) Relationship between median lifespan and carbohydrate:protein ratio (C:P) for results from (A). Median lifespan is shown (solid points) and whiskers represent the interquartile range (1st and 3rd quartiles). Whiskers may be slightly offset horizontally for clarity.
Figure 2
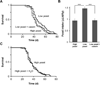
Dietary casein mimics the effect of yeast extract on fly lifespan. (A) Dahomey males on a high yeast diet (5% yeast extract + 5% sucrose) are short-lived compared to those maintained on a low yeast diet (1% yeast extract + 5% sucrose; p = 0.002, log-rank). This effect is mimicked by supplementing the low yeast diet with casein (2%, w/v) to match the final protein content of the high yeast food (p = 0.0015, log-rank). There was no significant difference in lifespan between flies maintained on the high yeast and low yeast + casein diets (p = 0.73, log-rank). N = 84 (low yeast), 76 (high yeast), and 87 (low yeast + casein). (B) Food intake over 24 hours on high yeast, low yeast, and low yeast + casein diets. Ingestion by Dahomey males was determined using the radioisotope labeling method. Increased yeast concentration (high yeast diet) or the equivalent amount of protein (low yeast + casein) results in reduced consumption compared to that on low yeast food (***, p < 0.001; one-way ANOVA followed by Tukey post-hoc test). No difference in food intake was seen between the high yeast and low yeast + casein diets (p > 0.05, Tukey post-hoc). Results shown are averages ± s.d. of N = 6 vials of 10–13 flies each. (C) Observed lifespan differences are not due to dehydration. Lifespan of Dahomey males on a high yeast diet was measured with (N= 61) and without (N = 59) access to an independent water source. No difference in lifespan was observed (p = 0.96, log-rank).
Figure 3
Relationship between dietary concentration of a nutrient and its consumption. Actual consumption of nutrients can differ greatly from predictions based solely on dietary composition. As (A) caloric and (B) carbohydrate content of the diet increases, actual consumption of calories and carbohydrate, respectively, does not concurrently increase. (C) Protein content of the diet shows a positive trend with actual protein intake, although the correlation is statistically insignificant. Two-tailed p-values for the Pearson correlation coefficient are shown along with a linear trendline. All data are for Canton-S males.
Figure 4
Regulation of feeding rate by dietary nutrient concentration. (A) Food intake over 24 hours on radiolabeled medium (average ± s.d.). Consumption is reduced with increasing levels of sucrose and yeast extract (S and Y, respectively, all shown as w/v), except at the highest concentration of sucrose (20%), where the effect of yeast is muted. All pairwise comparisons are significantly different (p < 0.001, one-way ANOVA followed by Tukey post-hoc test) except between the three diets with 20% sucrose (p > 0.05). (B) Relationship between food intake volume and macronutrient content of the diet. The size and darkness of the circles are scaled with increasing food intake. In general, increasing the concentration of dietary protein or carbohydrate reduces intake. However, conditions can be found where a change in the concentration of a single nutrient does not trigger compensatory feeding. (C) Consumption shows a strong negative correlation with caloric value of the diet, best described by a power model, suggesting an inverse relationship between the two parameters (y = m/xb; m = 0.40, b = 1.39). The power fit and R2 value are shown. When b = 1, the product of food intake and dietary caloric content is a constant, m, which describes a daily caloric intake target. When b > 1, over-feeding occurs as the caloric value of the diet decreases. The non-linear relationship between food intake and dietary caloric content was also tested using Spearman’s correlation coefficient and confirmed a strong negative correlation (r = −0.90, p = 0.005). All results are for Canton-S males.
Similar articles
- Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster.
Jensen K, McClure C, Priest NK, Hunt J. Jensen K, et al. Aging Cell. 2015 Aug;14(4):605-15. doi: 10.1111/acel.12333. Epub 2015 Mar 23. Aging Cell. 2015. PMID: 25808180 Free PMC article. - Mapping sex differences in the effects of protein and carbohydrates on lifespan and reproduction in Drosophila melanogaster: is measuring nutrient intake essential?
Carey MR, Archer CR, Rapkin J, Castledine M, Jensen K, House CM, Hosken DJ, Hunt J. Carey MR, et al. Biogerontology. 2022 Feb;23(1):129-144. doi: 10.1007/s10522-022-09953-2. Epub 2022 Feb 5. Biogerontology. 2022. PMID: 35122572 Free PMC article. - Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster.
Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Skorupa DA, et al. Aging Cell. 2008 Aug;7(4):478-90. doi: 10.1111/j.1474-9726.2008.00400.x. Epub 2008 Jun 28. Aging Cell. 2008. PMID: 18485125 Free PMC article. - Nutrient control of Drosophila longevity.
Tatar M, Post S, Yu K. Tatar M, et al. Trends Endocrinol Metab. 2014 Oct;25(10):509-17. doi: 10.1016/j.tem.2014.02.006. Epub 2014 Mar 28. Trends Endocrinol Metab. 2014. PMID: 24685228 Free PMC article. Review. - The impact of low-protein high-carbohydrate diets on aging and lifespan.
Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A, de Cabo R, Raubenheimer D, Simpson SJ. Le Couteur DG, et al. Cell Mol Life Sci. 2016 Mar;73(6):1237-52. doi: 10.1007/s00018-015-2120-y. Epub 2015 Dec 30. Cell Mol Life Sci. 2016. PMID: 26718486 Free PMC article. Review.
Cited by
- The Multifaceted Role of Nutrient Sensing and mTORC1 Signaling in Physiology and Aging.
Fernandes SA, Demetriades C. Fernandes SA, et al. Front Aging. 2021 Aug 27;2:707372. doi: 10.3389/fragi.2021.707372. eCollection 2021. Front Aging. 2021. PMID: 35822019 Free PMC article. Review. - Aging and energetics' 'Top 40' future research opportunities 2010-2013.
Allison DB, Antoine LH, Ballinger SW, Bamman MM, Biga P, Darley-Usmar VM, Fisher G, Gohlke JM, Halade GV, Hartman JL, Hunter GR, Messina JL, Nagy TR, Plaisance EP, Powell ML, Roth KA, Sandel MW, Schwartz TS, Smith DL, Sweatt JD, Tollefsbol TO, Watts SA, Yang Y, Zhang J, Austad SN. Allison DB, et al. F1000Res. 2014 Sep 12;3:219. doi: 10.12688/f1000research.5212.1. eCollection 2014. F1000Res. 2014. PMID: 25324965 Free PMC article. Review. - Identification of genetic modifiers of lifespan on a high sugar diet in the Drosophila Genetic Reference Panel.
Patel SP, Talbert ME. Patel SP, et al. Heliyon. 2021 Jun 5;7(6):e07153. doi: 10.1016/j.heliyon.2021.e07153. eCollection 2021 Jun. Heliyon. 2021. PMID: 34141921 Free PMC article. - Using Mouse and Drosophila Models to Investigate the Mechanistic Links between Diet, Obesity, Type II Diabetes, and Cancer.
Warr CG, Shaw KH, Azim A, Piper MDW, Parsons LM. Warr CG, et al. Int J Mol Sci. 2018 Dec 18;19(12):4110. doi: 10.3390/ijms19124110. Int J Mol Sci. 2018. PMID: 30567377 Free PMC article. Review. - Chronobiotics KL001 and KS15 Extend Lifespan and Modify Circadian Rhythms of Drosophila melanogaster.
Solovev IA, Shaposhnikov MV, Moskalev AA. Solovev IA, et al. Clocks Sleep. 2021 Aug 20;3(3):429-441. doi: 10.3390/clockssleep3030030. Clocks Sleep. 2021. PMID: 34449576 Free PMC article.
References
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 2005;6:298–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R15 AG027749/AG/NIA NIH HHS/United States
- 1R15AG027749/AG/NIA NIH HHS/United States
- R01 AG038012/AG/NIA NIH HHS/United States
- R21 DK092735/DK/NIDDK NIH HHS/United States
- AG038012/AG/NIA NIH HHS/United States
- R00AG030493/AG/NIA NIH HHS/United States
- R00 AG030493/AG/NIA NIH HHS/United States
- R01 AG031337/AG/NIA NIH HHS/United States
- R01 AG038688/AG/NIA NIH HHS/United States
- R21DK092735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases