Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex - PubMed (original) (raw)
Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex
Jochen Bauer et al. Neuropsychopharmacology. 2013 Jul.
Abstract
The upregulation of glutamatergic excitatory neurotransmission is thought to be partly responsible for the acute withdrawal symptoms and craving experienced by alcohol-dependent patients. Most physiological evidence supporting this hypothesis is based on data from animal studies. In addition, clinical data show that GABAergic and anti-glutamatergic drugs ameliorate withdrawal symptoms, offering indirect evidence indicative of glutamatergic hyperexcitability in alcohol-dependent subjects. We used proton magnetic resonance spectroscopy to quantify the glutamate (Glu) levels in healthy control subjects and in alcohol-dependent patients immediately after detoxification. The volumes of interest were located in the nucleus accumbens (NAcc) and the anterior cingulate cortex (ACC), which are two brain areas that have important functions in reward circuitry. In addition to Glu, we quantified the levels of combined Glu and glutamine (Gln), N-acetylaspartate, choline-containing compounds, and creatine. The Glu levels in the NAcc were significantly higher in patients than in controls. Craving, which was measured using the Obsessive Compulsive Drinking Scale, correlated positively with levels of combined Glu and Gln in the NAcc and in the ACC. The levels of all other metabolites were not significantly different between patients and controls. The increased Glu levels in the NAcc in alcohol-dependent patients shortly after detoxification confirm the animal data and suggest that striatal glutamatergic dysfunction is related to ethanol withdrawal. The positive correlation between craving and glutamatergic metabolism in both key reward circuitry areas support the hypothesis that the glutamatergic system has an important role in the later course of alcohol dependence with respect to abstinence and relapse.
Figures
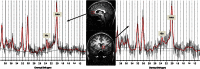
Figure 1
VOI locations in the bilateral rostral ACC (top) and in the left NAcc (bottom) with representative spectra in an alcohol-dependent patient.
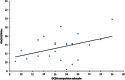
Figure 2
Correlation between the compulsion subscale of the Obsessive Compulsive Drinking Scale (OCDS) and the combined glutamate and glutamine (Glx) level in the nucleus accumbens (NAcc).
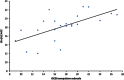
Figure 3
Correlation between the compulsion subscale of the Obsessive Compulsive Drinking Scale (OCDS) and the combined glutamate and glutamine (Glx) level in the anterior cingulate cortex (ACC).
Similar articles
- Perturbation of the glutamate-glutamine system in alcohol dependence and remission.
Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Thoma R, et al. Neuropsychopharmacology. 2011 Jun;36(7):1359-65. doi: 10.1038/npp.2011.20. Epub 2011 Mar 9. Neuropsychopharmacology. 2011. PMID: 21389979 Free PMC article. - Anterior Cingulate Glutamate Is Reduced by Acamprosate Treatment in Patients With Alcohol Dependence.
Frye MA, Hinton DJ, Karpyak VM, Biernacka JM, Gunderson LJ, Feeder SE, Choi DS, Port JD. Frye MA, et al. J Clin Psychopharmacol. 2016 Dec;36(6):669-674. doi: 10.1097/JCP.0000000000000590. J Clin Psychopharmacol. 2016. PMID: 27755217 Free PMC article. - Glutamate concentration in the anterior cingulate cortex in alcohol dependence: association with alcohol withdrawal and exploration of contribution from glutamatergic candidate genes.
Streit F, Treutlein J, Frischknecht U, Hermann D, Mann K, Kiefer F, Sack M, Hall ASM, Frank J, Witt SH, Foo JC, Degenhardt F, Heilmann-Heimbach S, Nöthen MM, Sommer WH, Spanagel R, Rietschel M, Ende G. Streit F, et al. Psychiatr Genet. 2018 Oct;28(5):94-95. doi: 10.1097/YPG.0000000000000202. Psychiatr Genet. 2018. PMID: 29985185 Free PMC article. - Neurobiological correlates of the disposition and maintenance of alcoholism.
Heinz A, Schäfer M, Higley JD, Krystal JH, Goldman D. Heinz A, et al. Pharmacopsychiatry. 2003 Nov;36 Suppl 3:S255-8. doi: 10.1055/s-2003-45139. Pharmacopsychiatry. 2003. PMID: 14677088 Review. - Glutamatergic plasticity and alcohol dependence-induced alterations in reward, affect and cognition.
Burnett EJ, Chandler LJ, Trantham-Davidson H. Burnett EJ, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:309-20. doi: 10.1016/j.pnpbp.2015.08.012. Epub 2015 Sep 1. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 26341050 Free PMC article. Review.
Cited by
- Quantitative electroencephalographic analysis of delirium tremens development following alcohol-withdrawal seizure based on a small number of male cases.
Yoon JE, Mo H, Kim DW, Im HJ. Yoon JE, et al. Brain Behav. 2022 Dec;12(12):e2804. doi: 10.1002/brb3.2804. Epub 2022 Oct 28. Brain Behav. 2022. PMID: 36306397 Free PMC article. - Brain Glutamate, GABA, and Glutamine Levels and Associations with Recent Drinking in Treatment-Naïve Individuals with Alcohol Use Disorder Versus Light Drinkers.
Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Prisciandaro JJ, et al. Alcohol Clin Exp Res. 2019 Feb;43(2):221-226. doi: 10.1111/acer.13931. Epub 2019 Jan 4. Alcohol Clin Exp Res. 2019. PMID: 30537347 Free PMC article. - Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice.
Griffin WC 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Griffin WC 3rd, et al. Neuropsychopharmacology. 2014 Feb;39(3):707-17. doi: 10.1038/npp.2013.256. Epub 2013 Sep 26. Neuropsychopharmacology. 2014. PMID: 24067300 Free PMC article. - The Role of Physical Exercise in Opioid Substitution Therapy: Mechanisms of Sequential Effects.
Psarianos A, Chryssanthopoulos C, Paparrigopoulos T, Philippou A. Psarianos A, et al. Int J Mol Sci. 2023 Mar 1;24(5):4763. doi: 10.3390/ijms24054763. Int J Mol Sci. 2023. PMID: 36902190 Free PMC article. Review. - Glutamine and GABA alterations in cingulate cortex may underlie alcohol drinking in a rat model of co-occurring alcohol use disorder and schizophrenia: an 1H-MRS study.
McCunn P, Chen X, Gimi B, Green AI, Khokhar JY. McCunn P, et al. Schizophrenia (Heidelb). 2022 Aug 23;8(1):67. doi: 10.1038/s41537-022-00272-6. Schizophrenia (Heidelb). 2022. PMID: 35999232 Free PMC article.
References
- Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29:1027–1034. - PubMed
- Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale. Arch Gen Psychiatry. 1996;53:225–231. - PubMed
- Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. - PubMed
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical