Microglia are essential to masculinization of brain and behavior - PubMed (original) (raw)
Microglia are essential to masculinization of brain and behavior
Kathryn M Lenz et al. J Neurosci. 2013.
Abstract
Brain sexual differentiation in rodents results from the perinatal testicular androgen surge. In the preoptic area (POA), estradiol aromatized from testosterone upregulates the production of the proinflammatory molecule, prostaglandin E(2) (PGE(2)) to produce sex-specific brain development. PGE(2) produces a two-fold greater density of dendritic spines in males than in females and masculinizes adult copulatory behavior. One neonatal dose of PGE(2) masculinizes the POA and behavior, and simultaneous treatment with an inhibitor of additional prostaglandin synthesis prevents this masculinization, indicating a positive feedforward process that leads to sustained increases in PGE(2). The mechanisms underlying this feedforward process were unknown. Microglia, the primary immunocompetent cells in the brain, are active neonatally, contribute to normal brain development, and both produce and respond to prostaglandins. We investigated whether there are sex differences in microglia in the POA and whether they influence developmental masculinization. Neonatal males had twice as many ameboid microglia as females and a more activated morphological profile, and both estradiol and PGE(2) masculinized microglial number and morphology in females. Microglial inhibition during the critical period for sexual differentiation prevented sex differences in microglia, estradiol-induced masculinization of dendritic spine density, and adult copulatory behavior. Microglial inhibition also prevented the estradiol-induced upregulation of PGE(2), indicating that microglia are essential to the feedforward process through which estradiol upregulates prostaglandin production. These studies demonstrate that immune cells in the brain interact with the nervous and endocrine systems during development, and are crucial for sexual differentiation of brain and behavior.
Figures
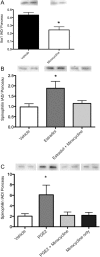
Figure 1.
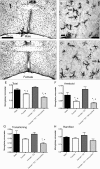
Sex differences and hormonal dependence of microglia in the POA. Iba1 staining in the POA on postnatal day 2 of a male pup (A, B) and a female pup (C, D) at 4× magnification and 20× magnification. Scale bar: A, B, 500 μm; C, D, 50 μm. Black arrows indicate examples of ameboid microglia; gray arrows indicate examples of transitioning microglia; white arrows indicate examples of ramified microglia. Males had significantly more total microglia (E) and ameboid microglia specifically (F) in the POA on PN2 than females. Estradiol masculinized microglial counts in females, and cotreatment of females with estradiol and minocycline prevented this masculinization. Among transitioning microglia, females cotreated with minocycline and estradiol had significantly fewer cells than males or estradiol-treated females (G). *Significantly different from males (ANOVA: p < 0.05). +Significantly different from females + E2 (ANOVA: p < 0.05). There was no sex difference or hormonal dependence on the number of ramified microglia (H).
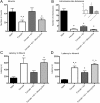
Figure 2.
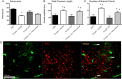
Three dimensional morphometric analysis of microglia (A–C). Males and estradiol-treated females had significantly larger microglial cell bodies (A), shorter processes (B), and fewer process branch points (C) than vehicle-treated females in the POA on PN2. Cotreatment with estradiol and minocycline prevented estradiol's effects on process length and number of branch points. *Significantly different from males (ANOVA: p < 0.05). +Significantly different from females + E2 (ANOVA: p < 0.05). D, Microglia in the neonatal POA do not stain for estrogen receptor α. Confocal imaging at 40× magnification of POA sections costained for tomato lectin (a pan-macrophage stain) and estrogen receptor α in males (pictured) and females show no colocalization. Scale bar, 150 μm. Arrows indicate examples of labeled microglia that are negative for estrogen receptor α staining.
Figure 3.
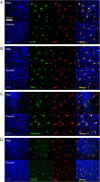
Microglia in the neonatal POA are positive for markers of classical (M1) and alternative (M2) microglial activation. A total of 100% of Iba1-stained microglia in males and females were positive for the M1 marker interleukin 1β (A), the M1 marker tumor necrosis factor α (B), the M2 marker arginase-1 (C), and the M2 marker CD206 (D). Confocal imaging performed at 40× magnification. Scale bar, 150 μm.
Figure 4.
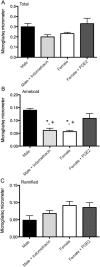
PGE2 induces ameboid microglial morphology in the POA. A, Effects of PGE2 and the COX inhibitor, indomethacin, on total microglial counts in the POA. B, Effects of PGE2 and indomethacin on ameboid microglial counts in the POA. Males had significantly more ameboid microglia in the POA on PN2 than females. Indomethacin prevented masculinization of ameboid counts in males and PGE2 masculinized microglial counts in females. *Significantly different from vehicle males (ANOVA; p < 0.05). +Significantly different from PGE2-treated females (ANOVA; p < 0.05). There was no sex difference or prostaglandin dependence on ramified microglia (C).
Figure 5.
Effects of microglial inhibition on dendritic spine-related proteins in vivo. A, The dose of minocycline used for all in vivo experiments (0.2 μg i.c.v.) significantly decreased levels of the microglial-associated protein Iba1 in the POA at PN2 relative to vehicle-treated controls. B, Estradiol treatment on PN0 and PN1 in females increased levels of the spine-related protein spinophilin in the POA by PN2 relative to vehicle-treated control females; coadministration of minocycline with estradiol prevented that increase in spinophilin. C, PGE2 produced similar increases in spinophilin to estradiol, and coadministration of PGE2 and minocycline similarly prevented this increase. Minocycline administration alone had no effect on spinophilin levels. *Significantly different from vehicle (ANOVA; p < 0.05).
Figure 6.
Effects of microglial inhibition on dendritic spine-related proteins in vitro. A, Representative images of MAP2 labeled neurons from primary POA cultures used to quantify dendritic spine-like protrusions at 100× magnification. Scale bar, 20 μm. B, Estradiol increased the density of spine-like protrusions relative to vehicle-treated controls. Estradiol-treated cultures depleted of microglia did not show an increase in protrusions, nor did cultures cotreated with estradiol and minocycline. There was no change in spine density in cultures treated only with minocycline. *Significantly different from vehicle (ANOVA; p < 0.05). C, Iba1 staining in control cultures and cultures depleted of microglia. Depleted cultures had ∼80% fewer microglia.
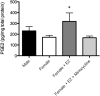
Figure 7.
Effects of microglial inhibition on PGE2 levels in the POA. Female pups treated with estradiol on PN0 and PN1 showed a significant increase in PGE2 levels in the POA at PN2 relative to vehicle-treated females, and cotreatment with estradiol and minocycline prevented this increase. *Significantly different from vehicle female (ANOVA; p < 0.05).
Figure 8.
Effects of neonatal microglial inhibition on adult masculine sex behavior. A, After adult gonadectomy and testosterone replacement, females had significantly fewer mounts relative to males, and neonatal estradiol treatment masculinized mount number. Cotreatment neonatally with estradiol and minocyline prevented this masculinization in females. B, All female groups showed significantly fewer intromission-like behaviors than males. When female groups were compared without males (inset), estradiol-treated females had an increased number of intromission-like behaviors than vehicle females, and cotreatment with minocycline prevented the estradiol-induced increase. C, Females had significantly longer latency to first mount than males; neonatal estradiol treatment masculinized latencies, and cotreatment neonatally with estradiol and minocyline prevented estradiol-induced masculinization in females. D, Control females had significantly longer latencies to first intromission-like behavior than males; estradiol-treated females had decreased latencies relative to vehicle-treated females, and minocycline prevented estradiol-induced decreases in latency to intromit. *Significantly different from male (p < 0.05). +Significantly different from vehicle female (p < 0.05). ∧Significantly different from estradiol-treated female (p < 0.05).
Comment in
- Microglia maketh the male.
Welberg L. Welberg L. Nat Rev Neurosci. 2013 Apr;14(4):226. doi: 10.1038/nrn3473. Epub 2013 Mar 6. Nat Rev Neurosci. 2013. PMID: 23463268 No abstract available.
Similar articles
- Mast Cells in the Developing Brain Determine Adult Sexual Behavior.
Lenz KM, Pickett LA, Wright CL, Davis KT, Joshi A, McCarthy MM. Lenz KM, et al. J Neurosci. 2018 Sep 12;38(37):8044-8059. doi: 10.1523/JNEUROSCI.1176-18.2018. Epub 2018 Aug 7. J Neurosci. 2018. PMID: 30093566 Free PMC article. - Prostaglandin-E2: a point of divergence in estradiol-mediated sexual differentiation.
Todd BJ, Schwarz JM, McCarthy MM. Todd BJ, et al. Horm Behav. 2005 Dec;48(5):512-21. doi: 10.1016/j.yhbeh.2005.07.011. Epub 2005 Aug 26. Horm Behav. 2005. PMID: 16126205 - Prostaglandin E2-induced masculinization of brain and behavior requires protein kinase A, AMPA/kainate, and metabotropic glutamate receptor signaling.
Wright CL, McCarthy MM. Wright CL, et al. J Neurosci. 2009 Oct 21;29(42):13274-82. doi: 10.1523/JNEUROSCI.3603-09.2009. J Neurosci. 2009. PMID: 19846715 Free PMC article. - Cellular mechanisms of estradiol-mediated masculinization of the brain.
Schwarz JM, McCarthy MM. Schwarz JM, et al. J Steroid Biochem Mol Biol. 2008 Apr;109(3-5):300-6. doi: 10.1016/j.jsbmb.2008.03.012. Epub 2008 Mar 8. J Steroid Biochem Mol Biol. 2008. PMID: 18430566 Free PMC article. Review. - Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder.
McCarthy MM, Wright CL. McCarthy MM, et al. Biol Psychiatry. 2017 Mar 1;81(5):402-410. doi: 10.1016/j.biopsych.2016.10.004. Epub 2016 Oct 11. Biol Psychiatry. 2017. PMID: 27871670 Free PMC article. Review.
Cited by
- Microglial phospholipase D4 deficiency influences myelination during brain development.
Chiba T, Otani Y, Yamaguchi Y, Ishibashi T, Hayashi A, Tanaka KF, Yamazaki M, Sakimura K, Baba H. Chiba T, et al. Proc Jpn Acad Ser B Phys Biol Sci. 2016;92(7):237-54. doi: 10.2183/pjab.92.237. Proc Jpn Acad Ser B Phys Biol Sci. 2016. PMID: 27477458 Free PMC article. - Minocycline causes widespread cell death and increases microglial labeling in the neonatal mouse brain.
Strahan JA, Walker WH 2nd, Montgomery TR, Forger NG. Strahan JA, et al. Dev Neurobiol. 2017 Jun;77(6):753-766. doi: 10.1002/dneu.22457. Epub 2016 Oct 14. Dev Neurobiol. 2017. PMID: 27706925 Free PMC article. - The Gut-Microglia Connection: Implications for Central Nervous System Diseases.
Wang Y, Wang Z, Wang Y, Li F, Jia J, Song X, Qin S, Wang R, Jin F, Kitazato K, Wang Y. Wang Y, et al. Front Immunol. 2018 Oct 5;9:2325. doi: 10.3389/fimmu.2018.02325. eCollection 2018. Front Immunol. 2018. PMID: 30344525 Free PMC article. Review. - Do Microglial Sex Differences Contribute to Sex Differences in Neurodegenerative Diseases?
Kodama L, Gan L. Kodama L, et al. Trends Mol Med. 2019 Sep;25(9):741-749. doi: 10.1016/j.molmed.2019.05.001. Epub 2019 Jun 3. Trends Mol Med. 2019. PMID: 31171460 Free PMC article. Review. - Microglia and Neonatal Brain Injury.
Mallard C, Tremblay ME, Vexler ZS. Mallard C, et al. Neuroscience. 2019 May 1;405:68-76. doi: 10.1016/j.neuroscience.2018.01.023. Epub 2018 Jan 17. Neuroscience. 2019. PMID: 29352997 Free PMC article. Review.
References
- Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002b;14:904–910. - PubMed
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. - PubMed
- Bilousova TV, Dansie L, Ngo M, Aye J, Charles JR, Ethell DW, Ethell IM. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102. - PubMed
- Burudi EM, Régnier-Vigouroux A. Regional and cellular expression of the mannose receptor in the post-natal developing mouse brain. Cell Tissue Res. 2001;303:307–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 NS076327/NS/NINDS NIH HHS/United States
- T32NS007375/NS/NINDS NIH HHS/United States
- F32NS076327/NS/NINDS NIH HHS/United States
- T32 NS007375/NS/NINDS NIH HHS/United States
- R01MH52716/MH/NIMH NIH HHS/United States
- R01 MH052716/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources