Tripartite motif ligases catalyze polyubiquitin chain formation through a cooperative allosteric mechanism - PubMed (original) (raw)
Tripartite motif ligases catalyze polyubiquitin chain formation through a cooperative allosteric mechanism
Frederick C Streich Jr et al. J Biol Chem. 2013.
Abstract
Ligation of polyubiquitin chains to proteins is a fundamental post-translational modification, often resulting in targeted degradation of conjugated proteins. Attachment of polyubiquitin chains requires the activities of an E1 activating enzyme, an E2 carrier protein, and an E3 ligase. The mechanism by which polyubiquitin chains are formed remains largely speculative, especially for RING-based ligases. The tripartite motif (TRIM) superfamily of ligases functions in many cellular processes including innate immunity, cellular localization, development and differentiation, signaling, and cancer progression. The present results show that TRIM ligases catalyze polyubiquitin chain formation in the absence of substrate, the rates of which can be used as a functional readout of enzyme function. Initial rate studies under biochemically defined conditions show that TRIM32 and TRIM25 are specific for the Ubc5 family of E2-conjugating proteins and, along with TRIM5α, exhibit cooperative kinetics with respect to Ubc5 concentration, with submicromolar [S]0.5 and Hill coefficients of 3-5, suggesting they possess multiple binding sites for their cognate E2-ubiquitin thioester. Mutation studies reveal a second, non-canonical binding site encompassing the C-terminal Ubc5α-helix. Polyubiquitin chain formation requires TRIM subunit oligomerization through the conserved coiled-coil domain, but can be partially replaced by fusing the catalytic domain to GST to promote dimerization. Other results suggest that TRIM32 assembles polyubiquitin chains as a Ubc5-linked thioester intermediate. These results represent the first detailed mechanistic study of TRIM ligase activity and provide a functional context for oligomerization observed in the superfamily.
Figures
FIGURE 1.
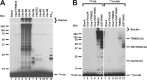
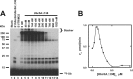
E2 screen for TRIM32-catalyzed 125I-ubiquitin conjugation. A, autoradiogram of SDS-PAGE-resolved assays of TRIM32-catalyzed 125I-ubiquitin chain formation. Assays were conducted as described under “Materials and Methods” in the presence of 44 n
m
Uba1, 50 n
m
of the indicated E2, and 800 n
m
TRIM32. B, autoradiogram of SDS-PAGE resolved assays conducted identically to panel A but in the presence of 1.6 μ
m
TRIM32 or 27 μ
m
GST-RING protein and 460 n
m
Ubc5A as indicated. Incubations additionally contained 5 μ
m
wild type (left subpanel) or reductively methylated (rmUB, right subpanel) 125I-ubiquitin. The left and right panels were exposed to normalize to account for the difference in specific radioactivity of the polypeptides. For panels A and B, migration positions for relative molecular weight standards are shown to the left. The position of the 5% stacker gel (Stacker), monoubiquitinated TRIM32 (Mwcalc = 80 kDa), monoubiquitinated GST-RING (Mwcalc = 44 kDa), monoubiquitinated Ubc5A (Mwcalc = 25 kDa), and free 125I-ubiquitin are shown to the right. Deviation of the apparent relative molecular weight of the monoubiquitinated species from their calculated molecular masses (Mwcalc) reflects non-ideality of ubiquitin due to its partial unfolding in SDS.
FIGURE 2.
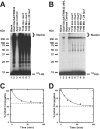
TRIM32 and TRIM32ΔNHL form free and conjugated polyubiquitin chains. Incubations identical to those of Fig. 1 were conducted with Ubc5A and either TRIM32 (panel A) or TRIM32ΔNHL (panel B) for 10 min then ATP was removed by the addition of apyrase as described under “Materials and Methods.” Following addition of human recombinant IsoT to a final concentration of 100 μ
m
, aliquots were taken at the indicated times. After 60 min an additional aliquot of IsoT was added and the reaction was incubated an additional 30 min. Migration positions for the relative molecular weight standards are shown to the left. Position of the 5% stacking gel and free 125I-ubiquitin are shown to the right. Panels C and D, total conjugated 125I-ubiquitin above 25 kDa, including the stacker gel, was quantified by associated radioactivity as described under “Materials and Methods” for the time points of panels A and B, respectively (closed circles). Open circles indicate conjugates remaining after addition of the second aliquot of IsoT. Data were fit to first order kinetics by nonlinear regression (solid line) to yield the end point (dashed line).
FIGURE 3.
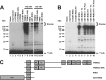
Polyubiquitin chain formation requires multimerization. A, incubations similar to those of Fig. 1 were conducted with TRIM32 (lanes 7 and 11), TRIM32ΔNHL (lanes 8 and 12), RING domain alone formed by processing with 90 IU/ml of thrombin for 30 min (lanes 9 and 13), or a GST-RING fusion (lanes 10 and 14). Reactions contained 48 n
m
Uba1 and either 450 (left subpanel) or 100 n
m
(right subpanel) of the indicated E3 protein in the absence (lanes 3–6) or presence (lanes 7–14) of 280 n
m
Ubc5A. B, thrombin-dependent processing of GST from the RING moiety is responsible for abrogation of chain formation. Conjugation reactions contained 34 n
m
Uba1 and 18 μ
m
GST-RING protein in the absence (lanes 3 and 4) or presence of 600 n
m
Ubc5A (lanes 4–10). The GST-RING or GST-RING ruined thrombin in which the thrombin cleavage site had been mutated to obviate processing were preincubated in the absence (lanes 5 and 8) or presence (lanes 6 and 9) of 90 IU/ml of thrombin for 30 min before addition to the conjugation assay. Alternatively, thrombin treatment was conducted after quenching the conjugation reaction (lanes 7 and 10). For both panels, migration positions for relative molecular weight standards are shown to the left. The position of the 5% stacking gel (Stacker), free 125I-ubiquitin, and mono-125I-ubiquitinated RING are shown to the right. C, schematic representation of constructs used in panels A and B.
FIGURE 4.
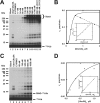
TRIM32 exhibits cooperativity with respect to [Ubc5A]o. A, autoradiogram of the dependence of TRIM32-catalyzed 125I-ubiquitin chain formation on [Ubc5A]o (lanes 4–11). To verify that reactions were E3 limiting, E1 and E3 concentrations were doubled in lanes 12 and 13, respectively, whereas holding the E2 concentration at 860 n
m
. Reactions contained 44 n
m
Uba1, the indicated concentrations of Ubc5A, and 80 n
m
TRIM32 protein, as indicated. The position of the 5% stacking gel and free 125I-ubiquitin are shown to the right. B, plot of the initial velocity of chain formation (the band in the stacking gel from panel A) versus [Ubc5A]o. The sigmoidal curve is a nonlinear least squares fit to the Hill equation of the data from lanes 4–9, prior to the observed substrate inhibition in lanes 10 and 11. The sigmoidicity of the plot is verified by the linearity of the corresponding Hill plot (inset). The substrate inhibition observed above 540 n
m
is indicated by the dashed line. C, autoradiogram of the dependence of RING domain-catalyzed auto-125I-monoubiquitination on [Ubc5A]o (lanes 4–8). Reactions contained 72 n
m
Uba1, the indicated concentrations of active Ubc5A, and 9.4 μ
m
RING domain protein, as indicated. The positions of the 5% stacking gel, RING-125I-ubiquitin, and free 125I-ubiquitin are shown on the right. The RING domain was generated prior to the assays by thrombin cleavage (20 IU of thrombin in 230 μl containing 118 μ
m
GST-RING fusion protein for 30 min at room temperature). D, plot of the initial velocity of RING auto-125I-monoubiquitination versus [Ubc5A]o. The hyperbolic shape of the curve was verified by the linearity of the double-reciprocal plot in the inset. The hyperbolic curve was derived by nonlinear least squares fit to the data in lanes 4–8, using GraFit (Erithacus Software Ltd.).
FIGURE 5.
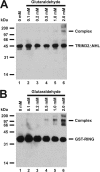
Glutaraldehyde cross-linking confirms oligomerization of TRIM32ΔNHL and GST-RING. Glutaraldehyde cross-linking reactions were conducted with either TRIM32ΔNHL (panel A) or GST-RING (panel B) proteins as described under “Materials and Methods” then resolved by 12% (w/v) SDS-PAGE before visualization by Western blotting. Migration positions for relative molecular weight standards are shown to the left.
FIGURE 6.
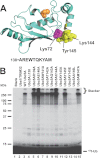
Mutations in the C-terminal α-helix of Ubc5A abrogate polyubiquitin chain formation. A, structure of Ubc5A with the active cysteine shown in orange spheres and the last 10 residues of the protein within the C-terminal α-helix are highlighted in yellow. The packing of Tyr145 between Lys72 and Lys146 is highlighted. The sequence for the C-terminal 10 residues is shown below the structure. The image was created from PDB file 2C4P (Dodd, R. B., and Read, R. J., unpublished coordinates) using PyMol (Schrodinger). B, autoradiogram comparing wild type Ubc5A (lane 3) and the indicated C-terminal point mutants (lanes 4–15) in their ability to support TRIM32-catalyzed 125I-ubiquitin chain formation. Reactions contained 64 n
m
Uba1, 100 n
m
active E2, and 200 n
m
TRIM32 protein, as indicated. Migration positions for relative molecular weight standards are shown to the left. The position of the 5% stacking gel and free 125I-ubiquitin are shown to the right.
FIGURE 7.
Ubc5AΔ138 supports allosteric cooperative activation of TRIM32. A, autoradiogram of the dependence of TRIM32-catalyzed polyubiquitin chain formation on the Ubc5AΔ138 concentration (lanes 5–14) in incubations containing 72 n
m
Uba1, 135 n
m
wild type Ubc5A, the indicated concentrations of Ubc5AΔ138, and 2.4 μ
m
TRIM32 protein as indicated. The positions of the 5% stacking gel and free 125I-ubiquitin are shown to the right. Migration positions for relative molecular weight standards are shown to the left. B, plot of the initial velocity for TRIM32-catalyzed 125I-ubiquitin chain formation versus [Ubc5AΔ138]o for the incubations in panel A as described under “Materials and Methods.”
FIGURE 8.
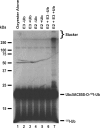
TRIM32 assembles polyubiquitin chains on Ubc5A. Autoradiogram of 80 n
m
purified Ubc5AC85S-125I-ubiquitin oxyester (lanes 1–7) incubated with 80 n
m
Uba1 (lanes 2–7) and, where indicated, 100 n
m
wild type Ubc5A, and 5 μ
m
unlabeled ubiquitin for 15 min at 37 °C before quenching with SDS sample buffer and SDS-PAGE resolution as described under “Materials and Methods.” Migration positions for relative molecular weight standards are shown to the left. The position of the 5% stacking gel, Ubc5AC85S-125I-ubiquitin oxyester, and free 125I-ubiquitin are shown to the right.
Similar articles
- In silico modeling of the cryptic E2∼ubiquitin-binding site of E6-associated protein (E6AP)/UBE3A reveals the mechanism of polyubiquitin chain assembly.
Ronchi VP, Kim ED, Summa CM, Klein JM, Haas AL. Ronchi VP, et al. J Biol Chem. 2017 Nov 3;292(44):18006-18023. doi: 10.1074/jbc.M117.813477. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924046 Free PMC article. - Functional role of TRIM E3 ligase oligomerization and regulation of catalytic activity.
Koliopoulos MG, Esposito D, Christodoulou E, Taylor IA, Rittinger K. Koliopoulos MG, et al. EMBO J. 2016 Jun 1;35(11):1204-18. doi: 10.15252/embj.201593741. Epub 2016 May 6. EMBO J. 2016. PMID: 27154206 Free PMC article. - RING Dimerization Links Higher-Order Assembly of TRIM5α to Synthesis of K63-Linked Polyubiquitin.
Yudina Z, Roa A, Johnson R, Biris N, de Souza Aranha Vieira DA, Tsiperson V, Reszka N, Taylor AB, Hart PJ, Demeler B, Diaz-Griffero F, Ivanov DN. Yudina Z, et al. Cell Rep. 2015 Aug 4;12(5):788-97. doi: 10.1016/j.celrep.2015.06.072. Epub 2015 Jul 23. Cell Rep. 2015. PMID: 26212332 Free PMC article. - Does it take two to tango? RING domain self-association and activity in TRIM E3 ubiquitin ligases.
Fiorentini F, Esposito D, Rittinger K. Fiorentini F, et al. Biochem Soc Trans. 2020 Dec 18;48(6):2615-2624. doi: 10.1042/BST20200383. Biochem Soc Trans. 2020. PMID: 33170204 Free PMC article. Review. - Orchestra for assembly and fate of polyubiquitin chains.
Kuhlbrodt K, Mouysset J, Hoppe T. Kuhlbrodt K, et al. Essays Biochem. 2005;41:1-14. doi: 10.1042/EB0410001. Essays Biochem. 2005. PMID: 16250894 Review.
Cited by
- Structural basis for the recognition and degradation of host TRIM proteins by Salmonella effector SopA.
Fiskin E, Bhogaraju S, Herhaus L, Kalayil S, Hahn M, Dikic I. Fiskin E, et al. Nat Commun. 2017 Jan 13;8:14004. doi: 10.1038/ncomms14004. Nat Commun. 2017. PMID: 28084320 Free PMC article. - Convergent evolution in the assembly of polyubiquitin degradation signals by the Shigella flexneri IpaH9.8 ligase.
Edwards DJ, Streich FC Jr, Ronchi VP, Todaro DR, Haas AL. Edwards DJ, et al. J Biol Chem. 2014 Dec 5;289(49):34114-28. doi: 10.1074/jbc.M114.609164. Epub 2014 Oct 23. J Biol Chem. 2014. PMID: 25342744 Free PMC article. - TRIM25 in the Regulation of the Antiviral Innate Immunity.
Martín-Vicente M, Medrano LM, Resino S, García-Sastre A, Martínez I. Martín-Vicente M, et al. Front Immunol. 2017 Sep 22;8:1187. doi: 10.3389/fimmu.2017.01187. eCollection 2017. Front Immunol. 2017. PMID: 29018447 Free PMC article. Review. - In silico modeling of the cryptic E2∼ubiquitin-binding site of E6-associated protein (E6AP)/UBE3A reveals the mechanism of polyubiquitin chain assembly.
Ronchi VP, Kim ED, Summa CM, Klein JM, Haas AL. Ronchi VP, et al. J Biol Chem. 2017 Nov 3;292(44):18006-18023. doi: 10.1074/jbc.M117.813477. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924046 Free PMC article. - Ubiquitin enzymes in the regulation of immune responses.
Ebner P, Versteeg GA, Ikeda F. Ebner P, et al. Crit Rev Biochem Mol Biol. 2017 Aug;52(4):425-460. doi: 10.1080/10409238.2017.1325829. Epub 2017 May 19. Crit Rev Biochem Mol Biol. 2017. PMID: 28524749 Free PMC article. Review.
References
- Chau V., Tobias J. W., Bachmair A., Marriott D., Ecker D. J., Gonda D. K., Varshavsky A. (1989) A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243, 1576–1583 - PubMed
- Pickart C. M. (2001) Mechanism underlying ubiquitination. Annu. Rev. Biochem. 70, 503–533 - PubMed
- Deshaies R. J., Joazeiro C. A. (2009) RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 - PubMed
- Haas A. L., Siepmann T. J. (1997) Pathways of ubiquitin conjugation. FASEB J. 11, 1257–1268 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous